TAMkin: A Versatile Package for Vibrational Analysis and Chemical Kinetics
Abstract
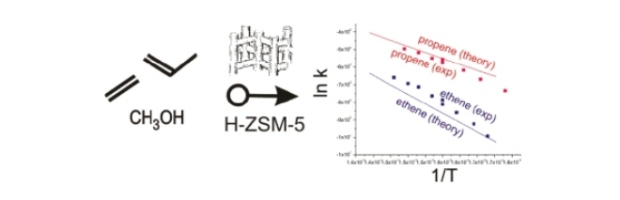
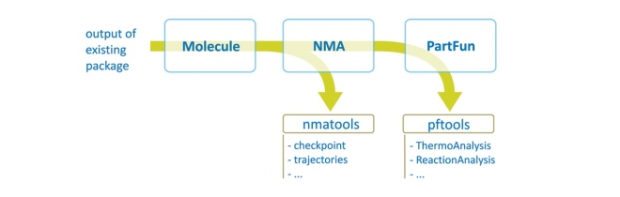
TAMkin is a program for the calculation and analysis of normal modes, thermochemical properties and chemical reaction rates. At present, the output from the frequently applied software programs ADF, CHARMM, CPMD, CP2K, Gaussian, Q-Chem, and VASP can be analyzed. The normal-mode analysis can be performed using a broad variety of advanced models, including the standard full Hessian, the Mobile Block Hessian, the Partial Hessian Vibrational approach, the Vibrational Subsystem Analysis with or without mass matrix correction, the Elastic Network Model, and other combinations. TAMkin is readily extensible because of its modular structure. Chemical kinetics of unimolecular and bimolecular reactions can be analyzed in a straightforward way using conventional transition state theory, including tunneling corrections and internal rotor refinements. A sensitivity analysis can also be performed, providing important insight into the theoretical error margins on the kinetic parameters. Two extensive examples demonstrate the capabilities of TAMkin: the conformational change of the biological system adenylate kinase is studied, as well as the reaction kinetics of the addition of ethene to the ethyl radical. The important feature of batch processing large amounts of data is highlighted by performing an extended level of theory study, which TAMkin can automate significantly.