Unraveling the Reaction Mechanisms Governing Methanol-to-Olefins Catalysis by Theory and Experiment

Abstract
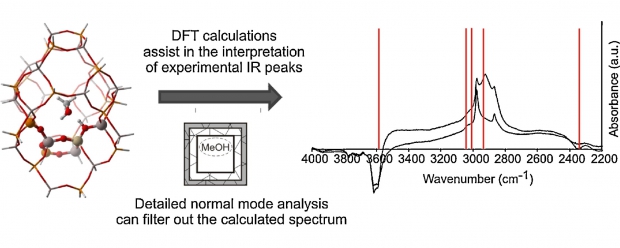
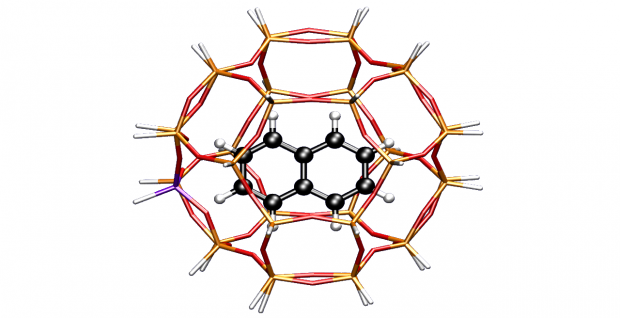
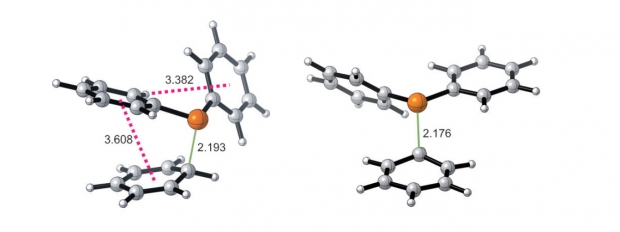
The conversion of methanol to olefins (MTO) over a heterogeneous nanoporous catalyst material is a highly complex process involving a cascade of elementary reactions. The elucidation of the reaction mechanisms leading to either the desired production of ethene and/or propene or undesired deactivation has challenged researchers for many decades. Clearly, catalyst choice, in particular topology and acidity, as well as the specific process conditions determine the overall MTO activity and selectivity; however, the subtle balances between these factors remain not fully understood. In this review, an overview of proposed reaction mechanisms for the MTO process is given, focusing on the archetypal MTO catalysts, H-ZSM-5 and H-SAPO-34. The presence of organic species, that is, the so-called hydrocarbon pool, in the inorganic framework forms the starting point for the majority of the mechanistic routes. The combination of theory and experiment enables a detailed description of reaction mechanisms and corresponding reaction intermediates. The identification of such intermediates occurs by different spectroscopic techniques, for which theory and experiment also complement each other. Depending on the catalyst topology, reaction mechanisms proposed thus far involve aromatic or aliphatic intermediates. Ab initio simulations taking into account the zeolitic environment can nowadays be used to obtain reliable reaction barriers and chemical kinetics of individual reactions. As a result, computational chemistry and by extension computational spectroscopy have matured to the level at which reliable theoretical data can be obtained, supplying information that is very hard to acquire experimentally. Special emphasis is given to theoretical developments that open new perspectives and possibilities that aid to unravel a process as complex as methanol conversion over an acidic porous material.

 Open Access version available at
Open Access version available at