H. Goossens, K. Vervisch, S. Catak, S. Stankovic, M. D'Hooghe, F. De Proft, P. Geerlings, N. De Kimpe, M. Waroquier, V. Van Speybroeck
Journal of Organic Chemistry
Abstract
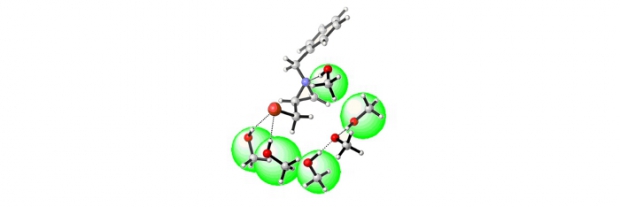
The difference in reactivity between the activated 2-bromomethyl-1-tosylaziridine and the non-activated 1-benzyl-2-(bromomethyl)aziridine with respect to sodium methoxide was analyzed by means of DFT calculations within the supermolecule approach, taking into account explicit solvent molecules. In addition, the reactivity of epibromohydrin with regard to sodium methoxide was assessed as well. The barriers for direct displacement of bromide by methoxide in methanol are comparable for all three heterocyclic species under study. However, ring opening was found to be only feasible for the epoxide and the activated aziridine, and not for the non-activated aziridine. According to these computational analyses, the synthesis of chiral 2-substituted 1-tosylaziridines can take place with inversion (through ring opening/ring closure) or retention (through direct bromide displacement) of configuration upon treatment of the corresponding 2-(bromomethyl)aziridines with one equivalent of a nucleophile, whereas chiral 2-substituted 1-benzylaziridines are selectively obtained with retention of configuration (via direct bromide displacement). Furthermore, the computational results showed that explicit accounting for solvent molecules is required to describe the free energy profile correctly. To verify the computational findings experimentally, chiral 1-benzyl-2-(bromomethyl)aziridines and 2-bromomethyl-1-tosylaziridines were treated with sodium methoxide in methanol. The presented work concerning the reactivity of 2-bromomethyl-1-tosylaziridine stands in contrast to the behaviour of the corresponding 1-tosyl-2-(tosyloxymethyl)aziridine with respect to nucleophiles, which undergoes a clean ring-opening/ring-closure process with inversion of configuration at the asymmetric aziridine carbon atom.