Stable Amorphous Solid Dispersion of Flubendazole with High Drug Loading via Solvent Electrospinning
Abstract
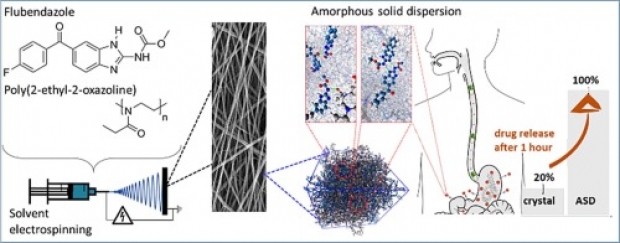
In this work, an important step is taken towards the bioavailability improvement of poorly water-soluble drugs, such as flubendazole (Flu), posing a challenge in the current development of many novel oral-administrable therapeutics. Solvent electrospinning of a solution of the drug and poly(2-ethyl-2-oxazoline) is demonstrated to be a viable strategy to produce stable nanofibrous amorphous solid dispersions (ASDs) with ultrahigh drug-loadings (up to 55 wt% Flu) and long-term stability (at least one year). Importantly, at such high drug loadings, the concentration of the polymer in the electrospinning solution has to be lowered below the concentration where it can be spun in absence of the drug as the interactions between the polymer and the drug result in increased solution viscosity. A combination of experimental analysis and molecular dynamics simulations revealed that this formulation strategy provides strong, dominant and highly stable hydrogen bonds between the polymer and the drug, which is crucial to obtain the high drug-loadings and to preserve the long-term amorphous character of the ASDs upon storage. In vitro drug release studies confirm the remarkable potential of this electrospinning formulation strategy by significantly increased drug solubility values and dissolution rates (respectively tripled and quadrupled compared to the crystalline drug), even after storing the formulation for one year.

 Open Access version available at
Open Access version available at