Origins of the Solvent Effect on the Propagation Kinetics of Acrylic Acid and Methacrylic Acid
Abstract
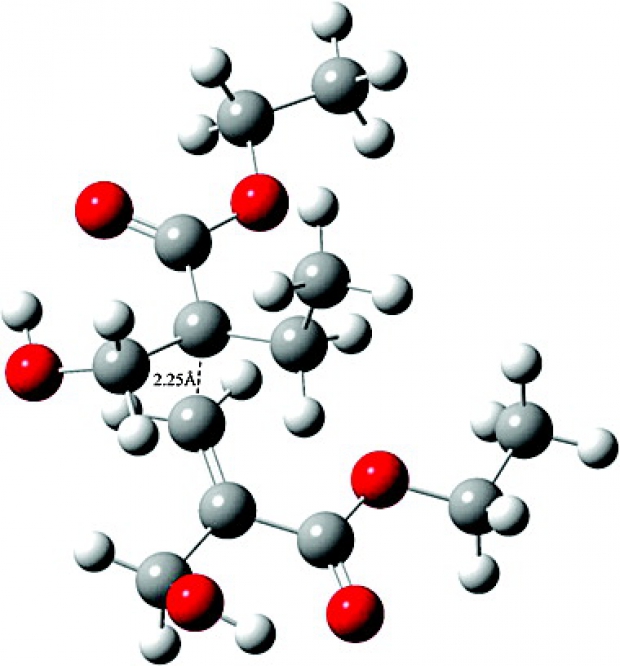
In this study, the relative rate of polymerization of acrylic acid (AA) versus methacrylic acid (MAA) and the effect of water on the polymerization kinetics are investigated within a combined static and molecular dynamics set of computational tools. Experimentally the relative rate of propagation of AA versus MAA is around 35 in bulk and 31 in water. Classical Molecular Dynamics calculations have been carried out to determine the location of the solvent molecules in the proximity of the dimeric poly(AA) and poly(MAA) units. A combined implicit/explicit solvent model was used for the evaluation of the kinetics of the dimeric polymer chains. We show that the rate acceleration of both polymers in water is mainly due to entropic rather than electrostatic effects and is in agreement with experimental findings. Moreover the slower propagation rate of MAA versus AA is ascribed to additional steric effects present in MAA due to the methyl group at the α position of the monomer. Among the functionals used, the M06-2X/6-311+G(3df,2p)//B3LYP/6-31+G(d) methodology reproduces the experimental rate constants quantitatively the best. © 2013 Wiley Periodicals, Inc. J Polym Sci Part A: Polym Chem, 2013