Abstract
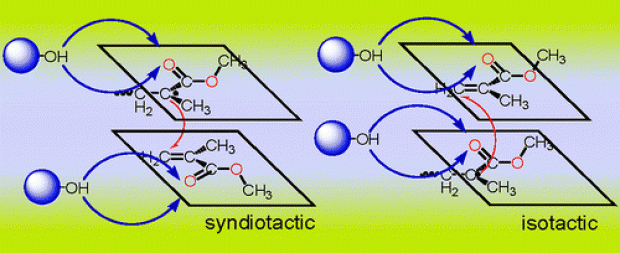
The control of stereochemistry in the free radical polymerization of methyl methacrylate (MMA) is important because the physical properties of PMMA are often significantly affected by the main-chain tacticity. In this study, the role of the solvent on the tacticity of MMA polymerization has been investigated by considering the propagation rate constants for the syndiotactic and isotactic free radical polymerization of MMA in vacuum, in methanol (CH3OH), and in 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol ((CF3)3COH). All geometry optimizations have been carried out with the B3LYP/6-31+G(d) methodology. The kinetics of the propagating dimer have been evaluated with the B3LYP/6-31+G(d), B3LYP/6-311+G(3df,2p), MPWB1K/6-311+G(3df,2p), and B2PLYP/6-31+G(d) methodologies. The role of the solvent has been investigated by using explicit solvent molecules and also by introducing a polarizable continuum model (IEF-PCM) with a dielectric constant specific to the solvent. Experimentally, the free radical polymerization of MMA in (CF3)3COH is found to be highly syndiotactic (rr = 75% at 20 °C): the stereoeffects of fluoroalcohols are claimed to be due to the hydrogen-bonding interaction of the alcohols with the monomers and growing species. This modeling study has revealed the fact that the solvents CH3OH and (CF3)3COH, which are H-bonded with the carbonyl oxygens located on the same side of the backbone hinder the formation of the isotactic PMMA to some extent. Methanol is less effective in reducing the isotacticity because of its small size and also because of the relatively loose hydrogen bonds (1.9 Å) with the carbonyl oxygens. The methodologies used in this study reproduce the solvent effect on the free radical polymerization kinetics of MMA in a satisfactory way.
