Theoretical study on the alteration of fundamental zeolite properties by methylene functionalization
Abstract
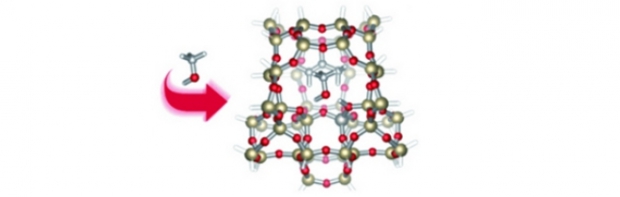
Following the recent boost of papers reporting synthesis of organic functionalized microporous and mesoporous materials, a detailed theoretical study was performed to probe the effect of organic functionalizations on certain fundamental properties in organosilicas from a microscopic viewpoint. The simplest functionalization of a bridging methylene unit was modeled in a zeolite MFI-type framework to serve as a model system for more complex organic moieties and other structures. Calculated adsorption energies for H2O and NH3 in methylenesilica reveal that the methylene functionalization increases the strength of the interaction of both probe molecules with the zeolite framework. Investigation of the combination of an ion-exchanged aluminum site containing a CH2-bridge demonstrates how the methylene moiety creates a steric obstruction for adsorbed alkali metal ions such as Li, Na and K, resulting in a weaker bond between these ions and the aluminum site. Finally, a study of proton mobility from a Brønsted acid site to a neighboring methylene bridge reveals that the acid proton will most likely migrate from the basic oxygen bridge to the methylene substitution. This implies that the combination of methylene moieties with aluminum impurities will lead to terminally bound methyl groups and cleavage of the hybrid organic–inorganic lattice.

 Open Access version available at
Open Access version available at