Abstract
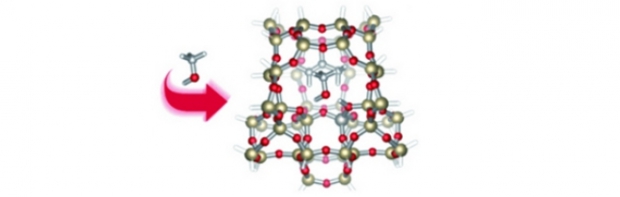
The methanol-to-olefin (MTO) process, catalyzed by acidic zeolites such as H-ZSM-5, provides an increasingly important alternative to the production of light olefins from crude oil. However, the various mechanistic proposals for methanol-to-olefin conversion have been strongly disputed for the past several decades. This work provides theoretical evidence that the experimentally suggested ‘alkene cycle’, part of a co-catalytic hydrocarbon pool, offers a viable path to the production of both propene and ethene, in stark contrast to the often- proposed direct mechanisms. This specific proposal hinges on repeated methylation reactions of alkenes, starting from propene, which occur easily within the zeolite environment. Subsequent cracking steps regenerate the original propene molecule, while also forming new propene and ethene molecules as primary products. Because the host framework stabilizes intermediate carbenium ions, isomerization and deprotonation reactions are extremely fast. Combined with earlier joint experimental and theoretical work on polymethylbenzenes as active hydrocarbon pool species, it is clear that, in zeolite H-ZSM-5, multiple parallel and interlinked routes operate on a competitive basis.
