
Abstract
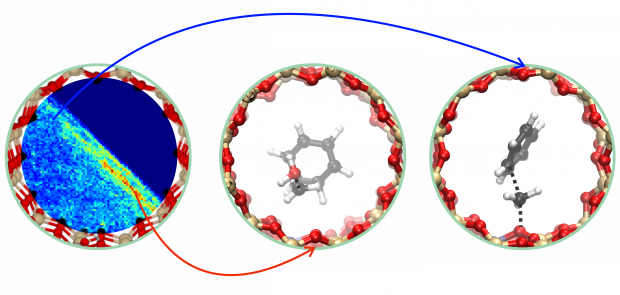
Zeolites are the workhorses of today’s chemical industry. For decades they have been successfully applied, however many features of zeolite catalysis are only superficially understood and in particular the kinetics and mechanism of individual reaction steps at operating conditions. Herein we use state-of-the-art advanced ab initio molecular dynamics techniques to study the influence of catalyst topology and acidity, reaction temperature and the presence of additional guest molecules on elementary reactions. Such advanced modeling techniques provide complementary insight to experimental knowledge as the impact of individual factors on the reaction mechanism and kinetics of zeolite-catalyzed reactions may be unraveled. We study key reaction steps in the conversion of methanol to hydrocarbons, namely benzene and propene methylation. These reactions may occur either in a concerted or stepwise fashion, i.e. methanol directly transfers its methyl group to a hydrocarbon or the reaction goes through a framework-bound methoxide intermediate. The DFT-based dynamical approach enables mimicking reaction conditions as close as possible and studying the competition between two methylation mechanisms in an integrated fashion. The reactions are studied in the unidirectional AFI-structured H-SSZ-24, H-SAPO-5 and TON-structured H-ZSM-22 materials. We show that varying the temperature, topology, acidity and number of protic molecules surrounding the active site may tune the reaction mechanism at the molecular level. Obtaining molecular control is crucial in optimizing current zeolite processes and designing emerging new technologies bearing alternative feedstocks.
 Open Access version available at UGent repository
Open Access version available at UGent repository