Angewandte Chemie int. Ed.
50 (50) 11990–11993
2011
A1

Abstract
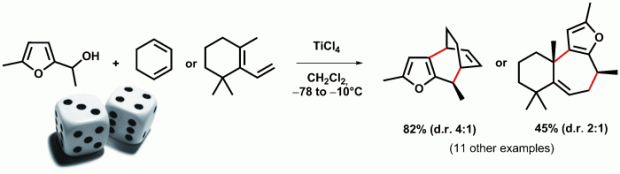
Furfuryl alcohols are revealed as direct reaction partners for a wide range of conjugated dienes in a (4+3) cycloaddition motif (see scheme). This novel Lewis-acid-promoted process gives straightforward access to various polycyclic skeletons containing a seven-membered ring. A plausible cationic stepwise mechanism was confirmed by DFT calculations.
