Abstract
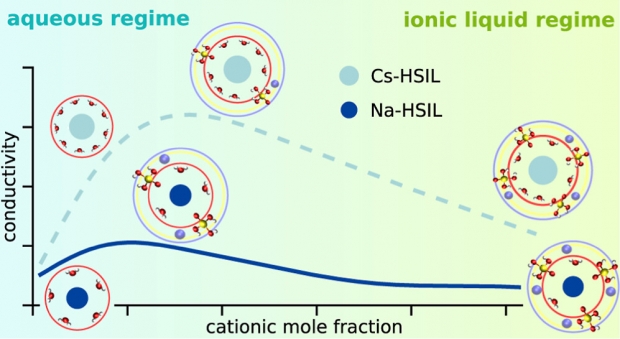
Despite the widespread use of zeolites in the chemical industry, their formation process is not fully understood due to the complex and heterogeneous structure of traditional synthesis media. Hydrated silicate ionic liquids (HSILs) have been proposed as an alternative. They are truly homogeneous and transparent mixtures with a low viscosity, facilitating experimental characterization. Interestingly, their homogeneous nature and simple speciation bring realistic molecular models of a zeolite growth liquid within reach for the first time. In this work, a simple molecular model is developed that gives insight into the crucial role of the alkali cations (sodium, potassium, rubidium, and cesium). Thereby, molecular dynamics simulations are combined with experimental measurements to demonstrate that the HSIL liquid structure strongly depends on the charge density and concentration of the alkali cation. As the water content increases, it transitions from a glassy network with fast ion exchange to an aqueous solution containing long-lasting solvated ion pairs. Furthermore, simulations reveal that the cation is capable of bringing several silicate monomers together in a glassy network, displaying perfect orientations for condensation reactions that underlie zeolite formation. This work is an important step toward the development of molecular models that can fully describe the early nucleation process of zeolites in combination with experiments.
 Open Access version available at UGent repository
Open Access version available at UGent repository