Abstract
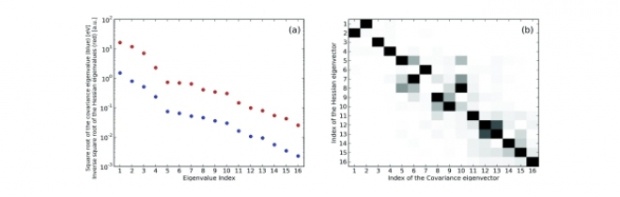
Charge equilibration models such as the electronegativity equalization method (EEM) and the split charge equilibration (SQE) are extensively used in the literature for the efficient computation of accurate atomic charges in molecules. However, there is no consensus on a generic set of optimal parameters, even when one only considers parameters calibrated against atomic charges in organic molecules. In this work, the origin of the disagreement in the parameters is investigated by comparing and analyzing six sets of parameters based on two sets of molecules and three calibration procedures. The resulting statistical analysis clearly indicates that the conventional least-squares cost function based solely on atomic charges is in general ill-conditioned and not capable of fixing all parameters in a charge-equilibration model. Methodological guidelines are formulated to improve the stability of the parameters. Although in this case a simple interpretation of individual parameters is not possible, charge equilibration models remain of great practical use for the computation of atomic charges.
