Abstract
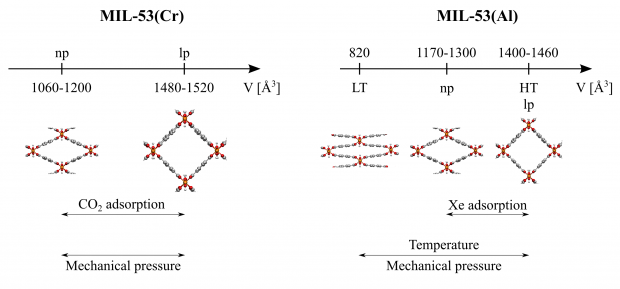
A new semi-analytical model is proposed to rationalize breathing of MIL-53 type materials. The model is applied on two case studies, the guest-induced breathing of MIL-53(Cr) with CO 2 and CH 4 , and the phase transformations for MIL-53(Al) upon xenon adsorption. Experimentally, MIL-53(Cr) breathes upon CO 2 adsorption, which was not observed for CH 4 . This result could be ascribed to the stronger interaction of carbon dioxide with the host matrix. For MIL-53(Al) a phase transition from the large pore phase could be enforced to an intermediate phase with volumes of about 1160 − 1300 A, which corresponds well to the phase observed experimentally upon xenon adsorption. Our thermodynamic model correlates nicely with the adsorption pressure model proposed by Coudert et al. Furthermore the model can predict breathing behavior of other flexible materials, if the user can determine the free energy of the empty host, the interaction energy between a guest molecule and the host matrix and the pore volume accessible to the guest molecules. This will allow to generate the osmotic potential from which the equilibria can be deduced and the anticipated experimentally observed phase may be predicted.
 Open Access version available at UGent repository
Open Access version available at UGent repository