Journal of Organic Chemistry
70 (9), 3674-3681
2005
A1
Abstract
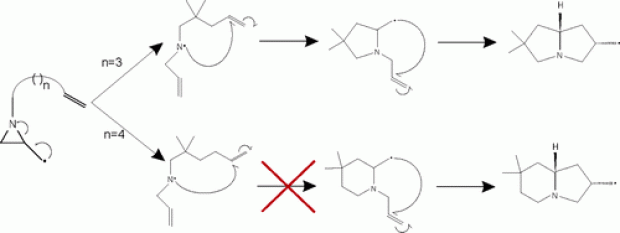
The radical cascade cyclizations of N-alkenyl-2-aziridinylmethyl radicals to pyrrolizidines and indolizidines were examined using density functional theory (DFT) calculations. A large preference for cyclization to pyrrolizidines was found. These predictions corroborated very well with experimental results, leading to an efficient synthesis of pyrrolizidines. No radical cascade cyclization to indolizidines could be performed in practice as only ring opening of N-alkenyl-2-aziridinylmethyl radicals to N-allyl-N-alkenylamines occurred.
