Abstract
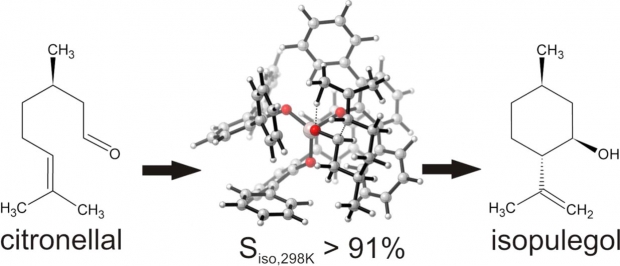
Industrial (-)-menthol production generally relies on the hydrogenation of (-)-isopulegol, which is in turn produced with high selectivity by cyclization of (+)-citronellal. This paper uses a combined theoretical and experimental approach to study the activity and selectivity of three Lewis acid catalysts for this reaction, namely ZnBr2, aluminum tris(2,6-diphenylphenoxide) (ATPH) and the heterogeneous metal-organic framework Cu3BTC2 (BTC = benzene-1,3,5-tricarboxylate). ATPH is a strong Lewis acid homogeneous catalyst with bulky ligands which provides very high selectivities for the desired stereo-isomer (> 99 %). The performance of the catalysts was evaluated as a function of temperature, which revealed that higher catalyst activity allows working at lower temperatures and improves the selectivity for isopulegol. The selectivity distribution is kinetically driven for ZnBr2 and ATPH. The theoretical selectivity distributions rely on the determination of an extensive set of diastereomeric transition states, for which the differences in free energy have been calculated using a complementary set of ab initio techniques. Given the sensitivity of the selectivity to small Gibbs free energy differences, the agreement between experimental and theoretical selectivities is satisfactory. On basis of the obtained insights rational design of new catalysts may be obtained. As proof of concept, the hypothetical Cu3(BTC-(NO2)3)2 Lewis catalyst – in which each phenyl hydrogen of the BTC ligand is replaced by a nitro group - is predicted to be very selective.
 Open Access version available at UGent repository
Open Access version available at UGent repository