The Formation of trans-Fused Macrocycles from N3,N3′-Polymethylenebis(hydantoins) by Ring-Closing Metathesis
Abstract
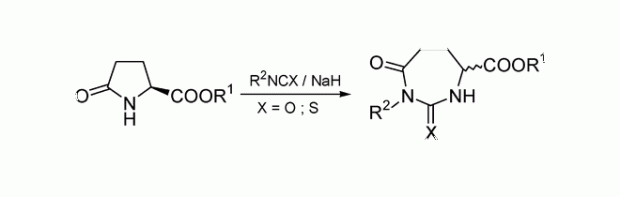
A straightforward ring transformation giving polymethylenebis(hydantoins) was extended, as these are HMBA analogues. Firstly, ethyl or allyl pyroglutamate was carbamoylated with a diisocyanate. Upon treatment with KOtBu in allyl alcohol the bis(carbamoyllactam) rearranged to give the hydantoin, which was followed by the ring-opening of the pyrrolidinone with formation of the allyl ester. These compounds were subsequently ring-closed in the presence of second-generation Grubbs' catalyst to form macrocycles containing the ester functionality in the ring. It was established by HSQC experiments with inverse detection that only the E isomers were formed in the cases of the 24- and 26-membered heterocycles. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2008)