Determination of the g Tensors for the Dominant Stable Radicals in X-Irradiated β-d-Fructose Single Crystals
Abstract
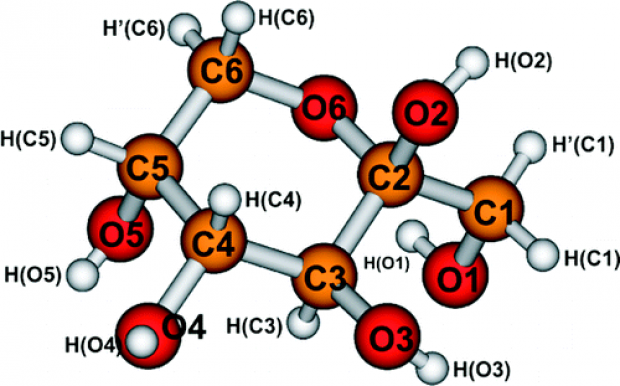
In spite of recent successful identifications of radicals produced after X-ray irradiation at 10 and 77 K in β-d-fructose, the structure of the two stable radicals dominating the electron paramagnetic resonance (EPR) spectrum after room temperature irradiation is still unclear. Based on the agreement between proton hyperfine (HF) tensors obtained in electron nuclear double resonance (ENDOR) experiments and the results of single molecule density functional calculations, a model for these radicals, involving OH abstraction at the C2 ring position, had previously been proposed, but this assignment could not be confirmed when the radical was embedded in a crystal environment. In this paper, we therefore provide additional experimental information for these radicals. First, their g tensors are determined from angular dependent ENDOR-induced EPR experiments. The relatively large anisotropy of these tensors is indicative of delocalization of the unpaired electron onto a neighboring oxygen atom. Second, EPR spectra of fructose powders, selectively enriched in 13C on various ring positions, are presented, demonstrating that the HF interaction with the carbon atom C3 is larger than with the C2. Combining the g tensor, proton and 13C HF data, we conclude that the structure of the stable radicals differs strongly from that of intact molecules and that further advanced quantum chemical modeling will be required to fully identify them.

 Open Access version available at
Open Access version available at