Radiation-Induced Radicals in Glucose-1-phosphate. II. DFT Analysis of Structures and Possible Formation Mechanisms
Abstract
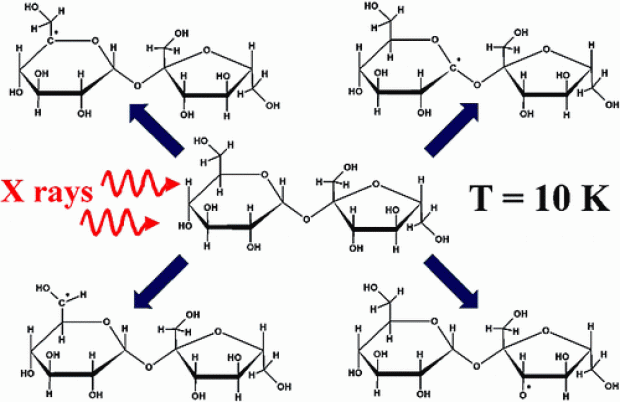
Four radiation-induced carbon-centered radicals in dipotassium glucose-1-phosphate dihydrate single crystals are examined with DFT methods, consistently relying on a periodic computational scheme. Starting from a set of plausible radical models, EPR hyperfine coupling tensors are calculated for optimized structures and compared with data obtained from EPR/ENDOR measurements, which are described in part I of this work. In this way, an independent structural identification is made of all the radicals that were observed in the experiments (R1−R4) and tentative reaction schemes are proposed. Also, the first strong evidence for conformational freedom in sugar radicals is established: two species are found to have the same chemical composition but different conformations and consequently different hyperfine coupling tensors. Analysis of the calculated energies for all model compounds suggests that the radiation chemistry of sugars, in general, is kinetically and not necessarily thermodynamically controlled.

 Open Access version available at
Open Access version available at