Ti-functionalized NH2-MIL-47: an effective and stable epoxidation catalyst
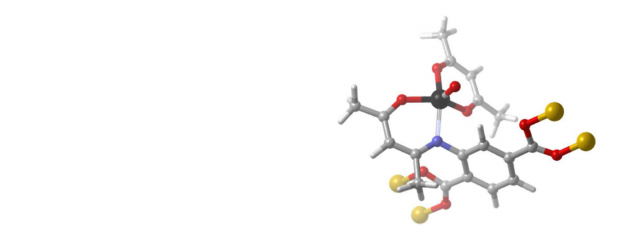
Abstract
In this paper, we describe the post-functionalization of a V-containing Metal-organic framework with TiO(acac)2 to create a bimetallic oxidation catalyst. The catalytic performance of this V/Ti-MOF was examined for the oxidation of cyclohexene using molecular oxygen as oxidant in combination with cyclohexanecarboxaldehyde as co-oxidant. A significantly higher cyclohexene conversion was observed for the bimetallic catalyst compared to the non-functionalized material. Moreover, the catalyst could be recycled at least 3 times without loss of activity and stability. No detectable leaching of V or Ti was noted. Electron paramagnetic resonance measurements were performed to monitor the fraction of V-ions in the catalyst in the +IV valence state. A reduction of this fraction by ∼17% after oxidation catalysis is observed, in agreement with the generally accepted mechanism for this type of reaction.
