Q-Band EPR and ENDOR of Low Temperature X-Irradiated β-d-Fructose Single Crystals
Abstract
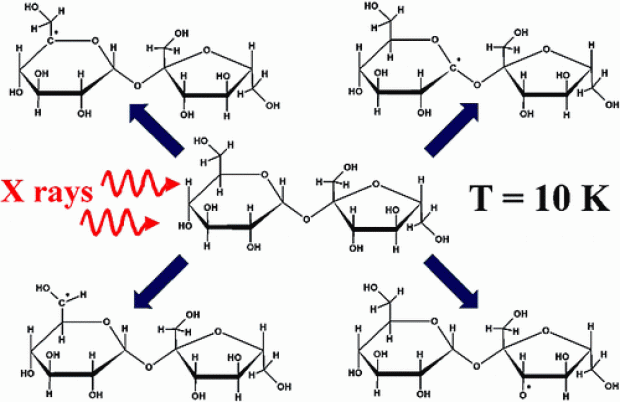
β-d-Fructose single crystals were in situ X-irradiated at 80 K and measured using electron paramagnetic resonance (EPR), electron nuclear double resonance (ENDOR) and ENDOR-induced EPR (EIE) techniques at Q-band (34 GHz) microwave frequencies. The measurements revealed the presence of at least four carbon-centered radicals stable at 80 K. By means of ENDOR angular variations in the three principal crystallographic planes, six proton hyperfine coupling tensors could be determined and were assigned to four different radicals by the aid of EIE. Two of the radicals exhibit only β-proton hyperfine couplings and reveal almost identical EIE spectra. For the other two radicals, the major hyperfine splitting originates from a single α-proton hyperfine coupling and their EIE spectra were also quite similar. The similarity of the EIE spectra and hyperfine tensors led to the assumption that there are only two essentially different radical structures. The radical exhibiting only β-proton hyperfine couplings was assigned to a C3 centered radical arising from H3 abstraction and the other radical suggested to be an open-ring species with a disrupted C2−C3 bond and a double C2−O2 bond. A possible formation mechanism for the latter open-ring radical is presented. By means of cluster density functional theory (DFT) calculations, the structures of the two radicals were determined and a fairly good agreement between the calculated and experimental hyperfine tensors was found.

 Open Access version available at
Open Access version available at