Acidity constant (pKa) calculation of large solvated dye molecules: evaluation of two advanced molecular dynamics methods
Abstract
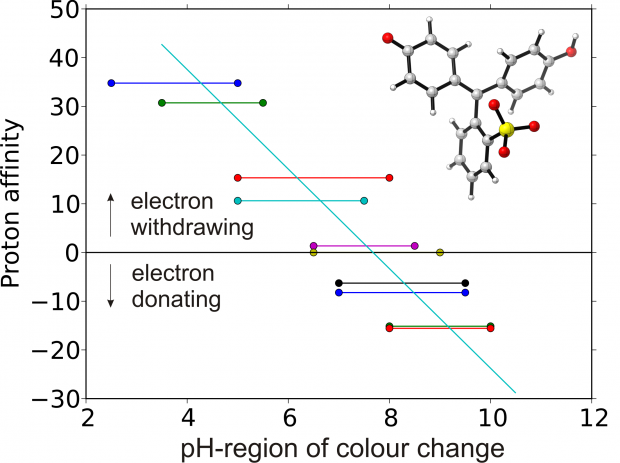
pH-sensitive dyes are increasingly applied onto polymer substrates for the creation of novel sensor materials. Recently, these dye molecules have been modified to form a covalent bond with the polymer host. This can have a large influence on the pH-sensitive properties, in particular on the acidity constant (pKa). Obtaining molecular control over the factors that influence the pK$_a$ value is mandatory for future intelligent design of sensor materials. Herein, we show that advanced molecular dynamics (MD) methods have reached the level where pKa values of large solvated dye molecules can be predicted with high accuracy. Two MD methods are used in this work: steered or restrained MD and the insertion/deletion scheme. Both are first calibrated on a set of phenol derivatives and afterwards applied to the dye molecule Bromothymol Blue. Excellent agreement with experimental values is obtained, which opens perspectives for using these methods for designing dye molecules.
 Open Access version available at UGent repository
Open Access version available at UGent repository