First principle chemical kinetics in zeolites: The Methanol-to-Olefin process as a case study
Abstract
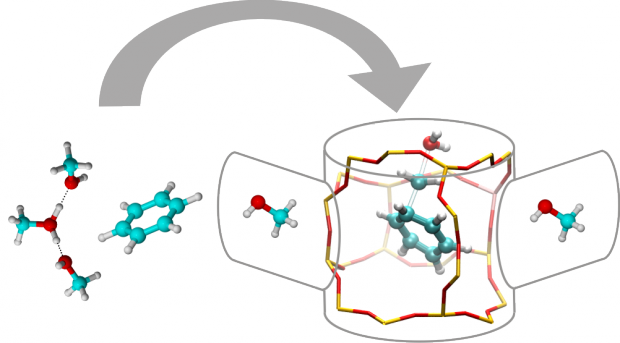
To optimally design next generation catalysts a thorough understanding of the chemical phenomena at the molecular scale is a prerequisite. Apart from qualitative knowledge on the reaction mechanism, it is also essential to be able to predict accurate rate constants. Molecular modeling has become a ubiquitous tool within the field of heterogeneous catalysis. Herein, we review current computational procedures to determine chemical kinetics from first principles, thus by using no experimental input and by modeling the catalyst and reacting species at the molecular level. Therefore, we use the methanol-to-olefin (MTO) process as a case study to illustrate the various theoretical concepts. This process is a showcase example where rational design of the catalyst was for a long time performed on the basis of trial and error, due to insufficient knowledge of the mechanism. For theoreticians the MTO process is particularly challenging as the catalyst has an inherent supramolecular nature, for which not only the Brønsted acidic site is important but also organic species, trapped in the zeolite pores, must be essentially present during active catalyst operation. All these aspects give rise to specific challenges for theoretical modeling. It is shown that present computational techniques have matured to a level where accurate enthalpy barriers and rate constants can be predicted for reactions occurring at a single active site. The comparison with experimental data such as apparent kinetic data for well-defined elementary reactions has become feasible as current computational techniques also allow predicting adsorption enthalpies with reasonable accuracy. Real catalysts are truly heterogeneous in a space- and time-like manner. Future theory developments should focus on extending our view towards phenomena occurring at longer length and time scales and integrating information from various scales towards a unified understanding of the catalyst. Within this respect molecular dynamics methods complemented with additional techniques to simulate rare events are now gradually making their entrance within zeolite catalysis. Recent applications have already given a flavor of the benefit of such techniques to simulate chemical reactions in complex molecular environments.
 Open Access version available at UGent repository
Open Access version available at UGent repository