Hydrogen transfer versus methylation: on the genesis of aromatics formation in the Methanol-To-Hydrocarbons over H-ZSM-5
Abstract
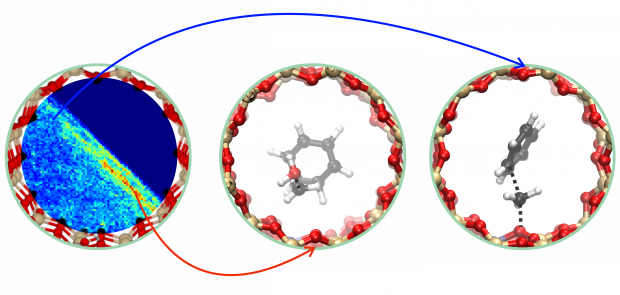
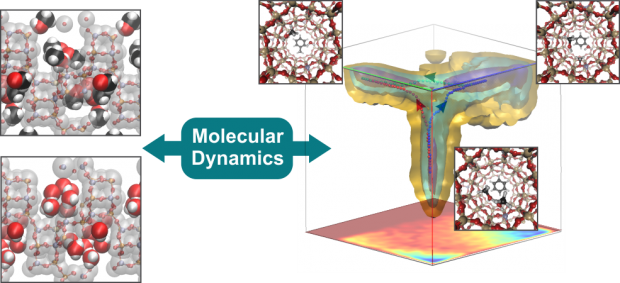
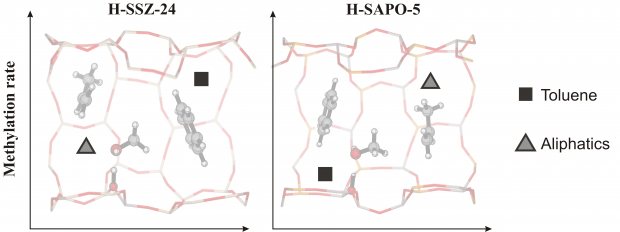
The catalytic conversion of methanol (MeOH) and dimethyl ether (DME) into fuels and chemicals over zeolites (MTH process) is industrially emerging as an alternative route to conventional oil-derived processes. After 40 years of research, a detailed mechanistic understanding of the intricate reaction network is still not fully accomplished. The overall reaction is described as two competitive catalytic cycles, dominated by alkenes and arenes, which are methylated and cracked or dealkylated to form effluent products. Herein, we present the reaction of isobutene with methanol and DME as an efficient tool for measuring the relative formation rates of alkenes and arenes, and we provide detailed mechanistic insight into the hydrogen-transfer reaction. We provide experimental and theoretical evidence that manifest a strong competition of methylation and hydrogen transfer of isobutene by methanol, while methylation is substantially favored by DME. Experiments performed at higher conversion facilitate projection of the results to the product distribution obtained when using MeOH or DME as feedstock during the MTH reaction.

 Open Access version available at
Open Access version available at