Zeolite-catalyzed biomass conversion in hot liquid water: how harsh reaction conditions can impact the working catalyst of future biorefineries
Zeolite-catalyzed biomass conversion in hot liquid water: how harsh reaction conditions can impact the working catalyst of future biorefineries
Promotor(en): V. Van Speybroeck /28127 / Nanoporous materialsBackground and problem
The massive exploitation of crude oil in the past decades has remarkably reduced the number of accessible oilfields around the globe. While this promotes the transition towards greener sources of energy, the impending shortage of cheap oil has negative implications for the chemical industry. Indeed, most carbon-based platform chemicals, such as aromatic monomers for plastic production, are directly obtained from crude oil fractions. For this reason, a lot of effort has been made in the past few years to develop a complete lignin-first biorefinery in which aromatics and other chemicals are obtained from renewable biomass [1]. Together with cellulose and hemicellulose, lignin is one of the main polymeric components of plant cell walls. In the paper production process, lignin is removed with aggressive depolymerization techniques and used as low value fuel. This however wastes a potentially profitable and renewable raw source of commodity chemicals, as lignin has the highest content by weight of aromatic units among natural polymers. In contrast, the lignin-first approach views lignin as a valuable component of the biomass and focuses on harvesting the molecular components. New catalysts and innovative processes have been developed to depolymerize lignin into a mixture of few phenolic monomers—including (alkyl)guaiacols—with high yield, leaving the cellulosic fraction almost unaffected for further usage [2].
Zeolites play a key role in the valorization of lignin-derived compounds, thanks to their stability under harsh reaction conditions, their micropore-induced selectivity, and all the general advantages of heterogenous catalysts. However, many reactions in lignin conversion would be ideally conducted in hot liquid water (HLW), an Achille’s heel of zeolite stability. This is the case for demethylation reactions conducted on lignin-derived (alkyl)guaiacols to obtain catechol, a common building block to produce bioplastics and other fine chemicals [3]. While some zeolite frameworks are known to withstand the harsh HLW conditions, it is now well established that (partial) hydrolysis of some bonds is inevitable. Specifically, the Al-O bonds of the zeolite active site are the most susceptible to hydrolysis, leading to the formation of (partially) hydrolyzed Al species [4]. How these species affect the catalytic process, possibly completely changing the reaction mechanism and intermediates with respect to “traditional” Brønsted acid sites, is so far not known. These microscopic effects are impossible to characterize experimentally, yet critical to understand and optimize the reaction conditions. Hence, a theoretical investigation will be performed to elucidate the still unknown effects of the (partially) hydrolyzed Al sites on guaiacol demethylation when HLW is present in zeolites (Figure 1).
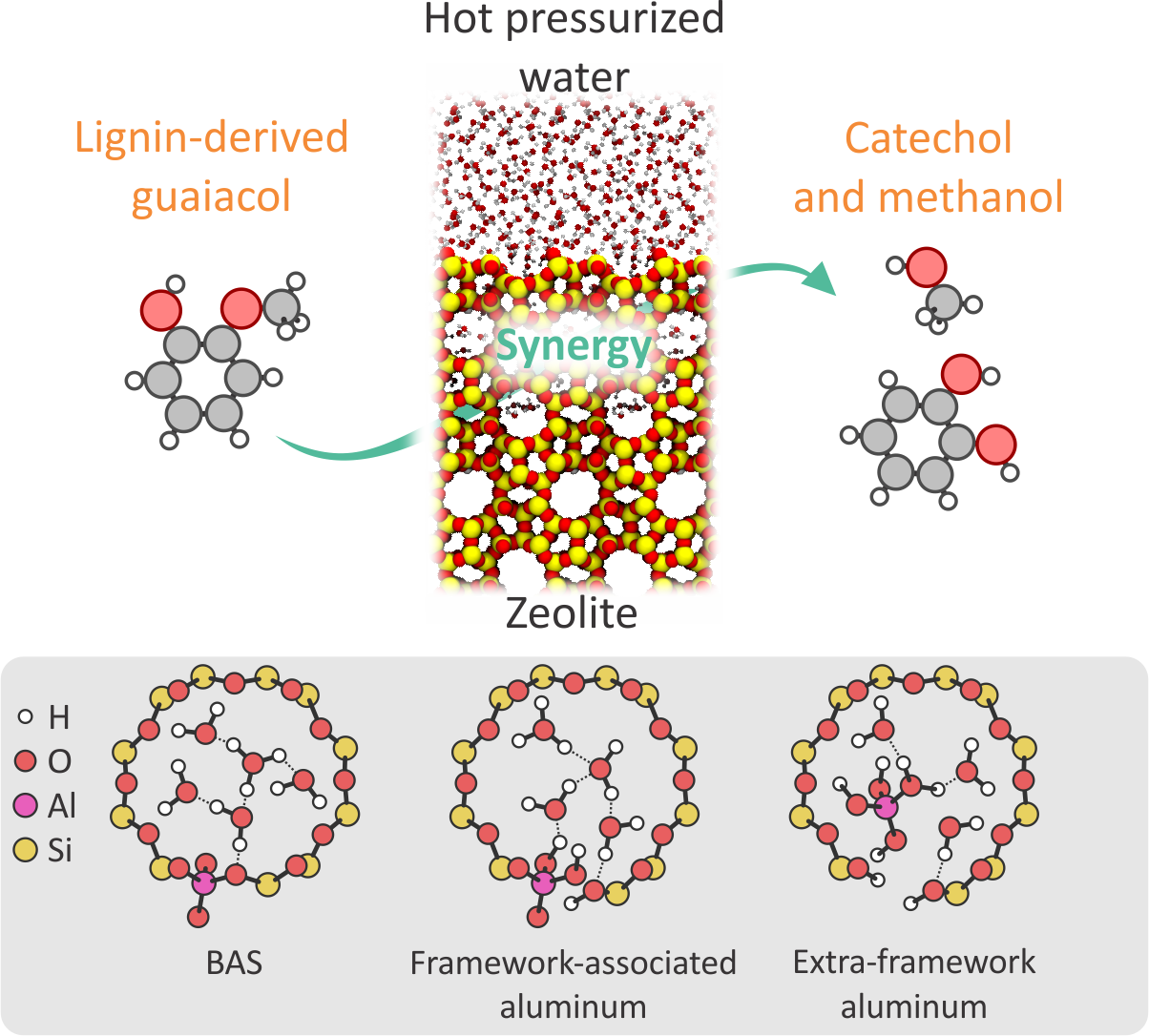
Figure 1: Schematic representation of guaiacol conversion in the synergistic zeolite + hot liquid water environment. On the bottom, some of the possible active sites that could be present in the working catalyst are shown.
Goal
The focus of this master thesis is a thorough understanding of the role water-induced defects play in demethylation reactions related to biomass conversion in the H-BETA zeolite, which has demonstrated efficacy in catalyzing this process [5]. Considering the harsh conditions at which this reaction is performed (liquid water, T>200°C, p>50bar) and the mobility of water molecules, the investigation will be performed with state-of-the-art molecular dynamics simulations at operating conditions. You will start by investigating the behavior and stability of various defective sites (regular Brønsted acid sites, framework-associated and extra-framework aluminum species) while hot pressurized water is present in the zeolite pores to understand which species are most likely to be present in the catalyst. Subsequently, the reactive guaiacol will be introduced into the zeolite to elucidate its influence on the water behavior as well as its interactions with the active site, expanding on previous investigations performed within our research group.
You will look for one or more possible dealkylation mechanisms and investigate them with enhanced sampling techniques. This will allow you to accurately quantify differences between the various possible active sites in terms of reaction barriers and reaction mechanisms, thus obtaining for the first time a complete overview on how they affect the reactivity of environmentally relevant molecules in water-filled zeolites. This information will be used to understand how catalytic efficiency evolves with time and guide process development.
The Center for Molecular Modeling (CMM) has an extensive experience in the study of zeolite-catalyzed reactions with advanced molecular simulations. You will be coached and guided when needed to become acquainted with the software and techniques required to study the systems of interest. The computational power necessary for the calculations will be ensured by CMM’s access to the main national supercomputing infrastructures. The proposed research topic is extremely relevant to current chemistry research; the experimental investigations of such reactions is ongoing. Resultantly, you will also have contact with the experimental group of Prof. Bert Sels (KU Leuven) and Prof. Bert Maes (UAntwerp), with whom the CMM has a consolidated collaboration.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, water, Biomass conversionReferences
[1] Schutyser, W. et al. Chem. Rev. 2018, 47, 852-908.
[2] Liao, Y. et al. Science 2020, 367, 1385-1390.
[3] Bomon, J. et al. Angew. Chem. Int. Ed. 2019, 132, 3087-3092.
[4] Heard, C. J. et al. Nat. Comm. 2019, 10, 4690.
[5] Wu, X. et al. ChemSusChem 2022, e202102248.
