Why water stops being a regular liquid: Understanding the structure of confined water in nanoporous ZIFs
Why water stops being a regular liquid: Understanding the structure of confined water in nanoporous ZIFs
Promotor(en): V. Van Speybroeck /20MODEV01 / Model and software developmentProbleemstelling:
Within the field of nanoporous materials [1], zeolites [2] and metal-organic frameworks (MOFs) [3, 4] form two classes of materials that are widely investigated, both computationally and experimentally, due to their intriguing properties. While inorganic zeolite frameworks are the workhorses in several industrial processes [5], MOFs are a more recent class of materials that holds great promise for applications such as gas storage and shock absorption. Given that MOFs are built up out of inorganic building blocks that are connected to one another through organic linkers, a virtually unlimited number of possible frameworks can be constructed out of the vast amount of building blocks. MOFs do however tend to be less stable than zeolites and most MOFs even decompose in the presence of water. Zeolites, on the other hand, show more limitations in the design and tunability [6], which restricts them to particular pore sizes and applications involving smaller molecules.
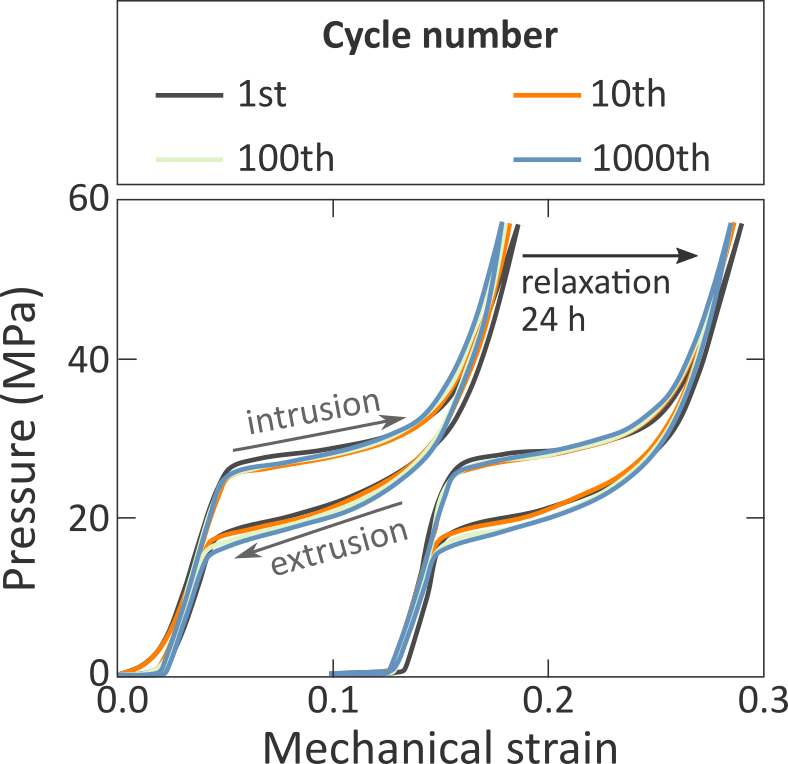
The so-called class of zeolitic imidazolate frameworks (ZIFs) uniquely combines the most attractive features of zeolites and MOFs. These materials make up a particular subset of MOFs with zeolite topologies, exhibiting an exceptional thermal and chemical stability. ZIFs are furthermore known to be stable in aqueous solutions and are inherently hydrophobic. For this reason, we recently started collaborating with the Tan group at the University of Oxford to investigate the feasibility of using ZIFs as shock absorbers, as their hydrophobicity endows them with a unique time-dependent response to the intrusion of liquids such as water [7]. For instance, when these materials are immersed in water and subjected to a mechanical shock, they show the typical intrusion/extrusion curve of Figure 1. Initially, the hydrophobicity of the ZIF prevents water from filling the pores of the material, until the exerted pressure is larger than the so-called intrusion pressure. At that pressure, water starts to intrude in the framework until the material is completely filled. Interestingly, when releasing the pressure, water remains adsorbed in the material until the pressure is decreased below the extrusion pressure, which for hydrophobic materials such as ZIFs is typically substantially lower than the intrusion pressure. Therefore, the hysteresis of this intrusion/extrusion curve results in a partial dampening of the initial shockwave, making ZIFs interesting materials for safety clothing or shock dampeners. To date, however, it remains unclear what fundamental phenomena form the basis for this interesting behaviour, precluding the practical use of ZIFs in safety devices.
Doelstelling:
As preliminary research in collaboration with the Tan group at the University of Oxford has indicated that the structure of water confined inside the ZIF’s pores plays a vital role in the intrusion/extrusion behaviour, the goal of this thesis is to arrive at a complete structural characterization of water confined within ZIFs. Several ZIF framework types, bearing different symmetries and topologies, will be investigated at different water loadings to investigate how the symmetry of the framework and the structure of the confined water influence the intrusion/extrusion behaviour of the material. This study will provide vital insight into which factors play a dominant role in the determination of the critical intrusion and extrusion pressures and how they influence the adsorption, organization, and dynamics of water inside the material’s pores. Besides the large expertise of the Center for Molecular Modeling (CMM) in the characterization of solvents in nanoporous materials, the proposed research will benefit from both national and international collaborations, including an ongoing collaboration with the experimental group of prof. Tan (University of Oxford) with whom we initiated the original research to design shock absorber materials.
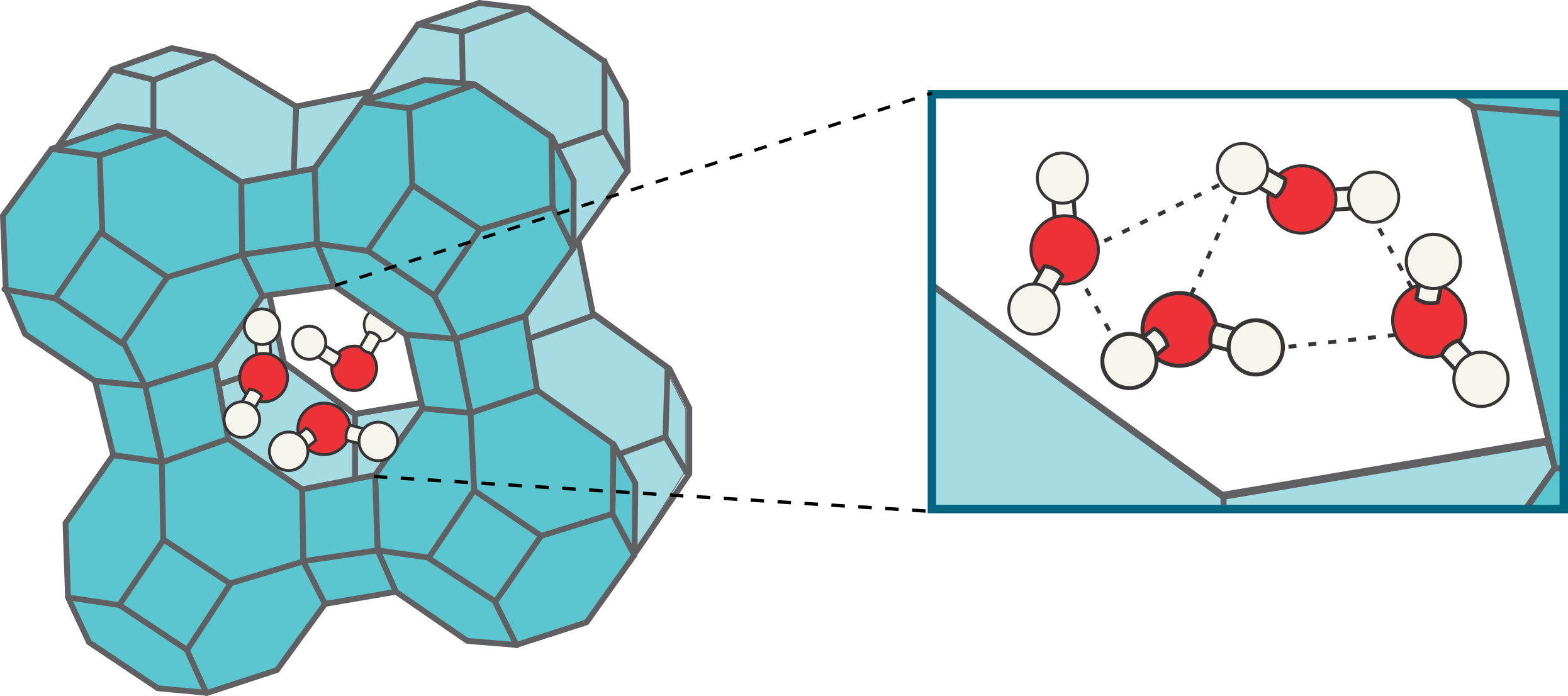
To obtain molecular-level insight into the intrusion/extrusion process, molecular dynamics (MD) simulations will be performed at a force field level, describing the interatomic interactions by means of an effective potential that is fitted to ab initio data obtained by solving the Schrödinger equation. In the MD simulations, the atomic nuclei are evolved throughout time by integrating Hamilton’s equations of motion. However, as a numerical integration of Hamilton’s equations of motion results in a sampling of the microcanonical (NVE) ensemble, extensions of the Hamiltonian description are necessary to be able to also sample the canonical (NVT) and isothermal-isobaric (NPT) ensembles, corresponding with actual, physical operating conditions. From the MD data, the structural organization of the confined water molecules can be determined at the realistic operating conditions. In ZIF-8, for instance, water is known to fill the cages sequentially by means of the formation of water clusters, as indicated schematically in Figure 2. The filling of a next cage is substantially facilitated by the interaction with water clusters that are already present [8]. Following this computational approach, it will be possible to elucidate the underlying principles determining the structural organization of water in ZIFs. As a result, the goal of this thesis is to provide rules of thumb on how ZIF materials can be tuned to optimize their performance as shock absorbers.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]KeywordsNanoporous materials, Molecular simulation, confined water, hydrogen bonding, topology, zeolitic imidazolate frameworksReferences
[1] A. G. Slater and A. I. Cooper, “Porous materials. Function-led design of new porous materials”, Science 348, 2015
[2] V. Van Speybroeck, K. Hemelsoet, L. Joos, M. Waroquier, R. G. Bell, and C. R. A. Catlow, “Advances in theory and their application within the field of zeolite chemistry”, Chem. Soc. Rev. 44, 7044-7111, 2015
[3] H. Furukawa, K. E. Cordova, M. O’Keeffe, and O. M. Yaghi, “The chemistry and applications of metal-organic frameworks”, Science 341, 1230444 (2013).
[4] S. Kitagawa, “Future porous materials”, Acc. Chem. Res. 50, 514–516 (2017).
[5] Y. Li, L. Li, and J. Yu, “Applications of Zeolites in Sustainable Chemistry”, Chem 3, 928-949 (2017).
[6] M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil, and V. Guillerm, “Zeolite-like metal-organic frameworks (ZMOFs): design, synthesis, and properties”, Chem. Soc. Rev. 44, 228-249 (2015).
[7] Y. Sun, Y. Li, and J.-C. Tan, “Framework flexibility of ZIF-8 under liquid intrusion: discovering time-dependent mechanical response and structure relaxation”, Phys. Chem. Chem. Phys. 20, 10108-10113 (2018).
[8] H. Zhang and R. Q. Snurr, “Computational Study of Water Adsorption in the Hydrophobic Metal-Organic Framework ZIF-8: Adsorption Mechanism and Acceleration of the Simulations”, J. Phys. Chem. C 121, 24000-24010 (2017).
