Useful and elegant atoms in molecules
Useful and elegant atoms in molecules
Promotor(en): T. Verstraelen, D. Van Neck /MM_14_MODEV_05 / Model and software development, Many-particle physicsThe (philosophical) concept of the atom goes back to ancient history when it was first introduced by Demokritos. Ever since the work of John Dalton, it is generally accepted that all matter is composed of atoms. This fundamental concept in chemistry allows one to describe molecules, reactions, … in terms of atomic elements. With the advent of quantum mechanics, it became also possible to predict the electronic structure of molecules and solids. Such computations do not rely on a definition of an atom in a molecule. There is not even a generally accepted definition of atoms in molecules (AIM). Nevertheless, partitioning the wavefunction into atomic contributions could immensely facilitate the physical or chemical interpretation of the electronic wavefunction. An example is given in the figure below where the electron density of the CO2 molecules is partitioned in atomic contributions. Over the past 60 years, many of these AIM schemes were proposed.
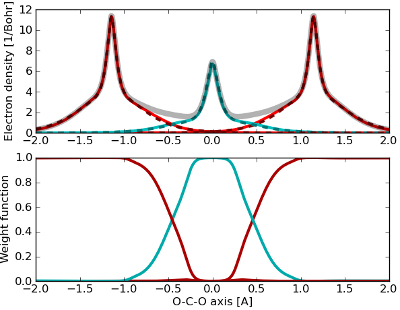
The definition of atomic partial charges is one of the main applications of AIM schemes. One may use these charges to model molecular electrostatics if the AIM scheme satisfies two criteria: (i) the charges should reproduce the electrostatic potential around the molecule, (ii) the charges should be robust with respect to computational details and conformational changes. In general, it is also desirable that the AIM scheme is mathematically elegant, such that its behavior can be characterized by solid theorems. Although these criteria can not always be measured objectively, it is clear that there is currently no AIM scheme in the literature that satisfies all these three criteria.
Several new AIM schemes are under active development at the Center for Molecular Modeling (CMM). The student will take actively part in this research by (i) making a critical analysis of the different methodologies, (ii) testing the new schemes on standard sets of test molecules and (iii) improving the mathematical definitions of the partitioning schemes. The student has of course the freedom to explore related topics such as the partitioning of electronic density matrices.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]KeywordsHirshfeld partitioning, Atomic partial charges, Information theory, Computational assessment, Computational physicsRecommended coursesComputational physics (C001827); Modeling and simulation at the nanoscale (E023370); Many-body physics (C001759)

