Transport modeling for novel organic solvent nanofiltration membranes
Transport modeling for novel organic solvent nanofiltration membranes
Promotor(en): V. Van Speybroeck /16NANO06 / Nanoporous materialsDow Water & Process Solutions is an important player in the membrane filtration market. Their membranes are used in water purification both for industrial purposes and to obtain drinking water. Nanofiltration (NF), where nanoporous membranes are used, is a very promising technique for liquid purification, as the pore size is small enough to retain salts and organic molecules, while the required pressures to operate are much smaller than for reverse osmosis [1]. Encouraged by these results, the principle of nanofiltration is now also emerging in the purification of industrial solvent streams. However, the introduction of organic solvents complicates the molecular interactions dramatically.
In these membrane filtration applications, the membrane needs to be chosen such that its nanopores hinder the transport of large organic molecules present in the stream, while the smaller molecules and the solvent, often water or acetone, can move freely through the membrane (see figure 1). Hence, information about the size of these molecules relative to the pores present in the membrane is needed. However, this is complicated by the various interplays between the solvent, solute and membrane. For instance, the radius of gyration of the solute, which determines its size, can be completely different in water than in acetone, since possible solvation of the solute basically causes solvent molecules to add to the effective radius of the solute. In this thesis, we will specifically focus on the diffusion of different polyethylene glycol (PEG) oligomers in water and acetone through a ceramic TiO2 membrane. Since the pores of this ceramic membrane can be assumed independent of the solvent, the main challenge is to understand the solvent effect on the diffusion of the PEG oligomers through the membrane. A thorough understanding of this effect, and hence the energies and barriers associated with it, is crucial to expand the nanofiltration applications of our industrial partner, Dow.

Goal
To elucidate the process with which the PEG oligomers may pass through the membrane and answer the aforementioned questions, simulations on the molecular level are indispensable. In these simulations, the effect of the solvent on the radius of gyration for the PEG oligomers, the structure of the TiO2 membrane, and the diffusive process of the PEGs through the membrane can be studied directly. Hence, the challenge is threefold.
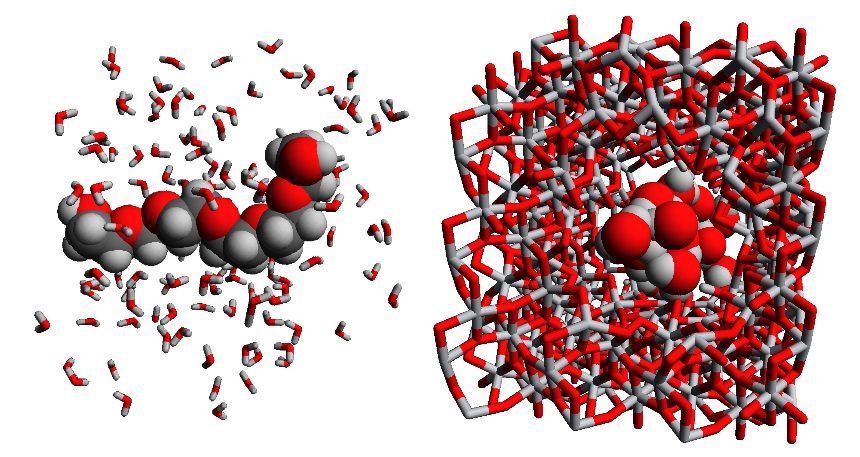
In a first step, different PEG oligomers will be studied in water or acetone, to obtain information on the solvent effect on the radius of gyration of the different PEG oligomers (see figure 2(left)). Crucial in this step is the accurate description of the interactions between the PEG oligomers and the solvent. However, since we are studying a large system (>100 atoms) on a time scale up to a few nanoseconds, a complete quantum mechanical description is not feasible. Hence, to determine the forces acting on each of the atoms during the molecular simulation, force fields will be used. In these force fields, the potential energy surface is approximated by an analytical expression to speed up the simulation and achieve a timescale of several nanoseconds. In this thesis, we will consider both force fields available in literature as well as new force fields derived based on our in-house protocol QuickFF [2]. At the end of this step, an accurate description of the PEG and solvent is necessary, so that the average radius of gyration of the PEG in this solvent can be determined. Moreover, the sensitivity of the results on the force field model and the periodic box size will be assessed.
In a second step, a TiO2 membrane needs to be modeled in the solvent. Here again, a force field needs to be taken from literature or derived via QuickFF. With this force field, the ceramic membrane can be optimized, and the distribution of pore sizes in the nanoporous material can be determined, for instance using Zeo++. Comparing this pore size with the PEG’s radius of gyration of the previous step will already allow the student to make a statement on the ease of diffusion and to distinguish between hindered diffusion, for which an energetic barrier hinders the diffusion through the pores, and free diffusion, without such barriers (see figure 2(right)).
In a third step, the force fields obtained in the previous steps will be combined to model the diffusion of the solvated PEG oligomers through the membrane. Depending on the results of the second step, three types of simulations can be performed. In case of free diffusion, regular molecular dynamics (MD) simulations will already yield accurate results. However, in case of hindered diffusion, more advanced techniques, such as restrained MD, need to be considered to accurately sample the diffusion process in a feasible time scale. A third, more abstract option, is to model the diffusion by means of a tube with a confining radial field, mimicking the membrane pores. Here, parametrization of the confining field critically depends on the results of the second step.
Combining the results of the three steps, the student will be able to answer glaring questions on the solvent effect on the diffusion of PEG oligomers through a ceramic membrane, and hence aid the computational design of membranes for nanofiltration applications. The CMM has ample expertise in modeling nanoporous materials, and is now expanding this knowledge towards membrane filtration. This work will be performed in active collaboration with Dow, which will introduce the student to the concept of nanofiltration. The student will be actively coached to get acquainted with all necessary techniques to perform the research successfully. The student will furthermore be actively involved in work discussions with our partners at Dow Terneuzen and several side visits will be possible.
Aspects
Physics aspect: Force field description of different classes of materials, study of diffusion through a membrane
Engineering aspect: Application of diffusion for nanofiltration.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) MODELING, MATERIALS, NANOKeywordsDiffusion, filtration, Force fields, Molecular dynamics, oligomers, Nanoporous materialsReferences
[1] A. W. Mohammad, Y. H. Teow, W. L. Ang, Y. T. Chung, D. L. Oatley-Radcliffe en N. Hilal, „Nanofiltration membranes review: Recent advances and future prospects,” Desalination, vol. 356, pp. 226-254, 2015.
[2] L. Vanduyfhuys, S. Vandenbrande, T. Verstraelen, R. Schmid, M. Waroquier en V. Van Speybroeck, „QuickFF: A program for a quick and easy derivation of force fields for metal-organic frameworks from ab initio input,” J. Comput. Chem., vol. 36, nr. 13, pp. 1015-1027, 2015.


