Temperature dependence of the free energy of nanoporous materials
Temperature dependence of the free energy of nanoporous materials
Promotor(en): V. Van Speybroeck /16NANO08 / Nanoporous materialsThe free energy of a macroscopic system is one of the most important thermodynamic quantities to describe the behavior the system. By definition it describes the amount of energy that is available to the system to perform work (hence, the amount of energy that is 'free') under a given set of conditions. For example under conditions of fixed number of particles (N), fixed volume (V) and fixed temperature (T), the Helmholtz free energy (F) is given by F=U-TS, in which U is the internal energy and S is the entropy. The internal energy, entropy and free energy are all state functions dependent on the temperature. This temperature dependence is crucial to understand several macroscopic properties such as thermal expansion and heat capacity. Although such temperature dependence is straightforward to describe in simple or ideal systems, such as the harmonic oscillator or the ideal gas, it is much more difficult in realistic systems.
Some systems show a very complex free energy profile, which may consist of various stable minima and which may be very prone to temperature effects. At the Center for Molecular Modeling we are investigating extensively Metal-organic frameworks. These are hybrid materials consisting of metal-oxide clusters interconnected by means of organic linkers, resulting in a 3D periodic framework with nanosized pores (nanoporous crystal). The discovery of metal-organic frameworks (MOFs) was a milestone in material science and computational physics. The framework of some of these materials (eg. MIL-53) is inherently very flexible, enabling them to change the size and shape of their unit cell under influence of external stimuli, such as pressure and adsorption of guest molecules, but also temperature. This special type of behavior is unique and is known in literature as framework breathing. To date despite numerous efforts of researchers, the breathing phenomenon is not fully understood.
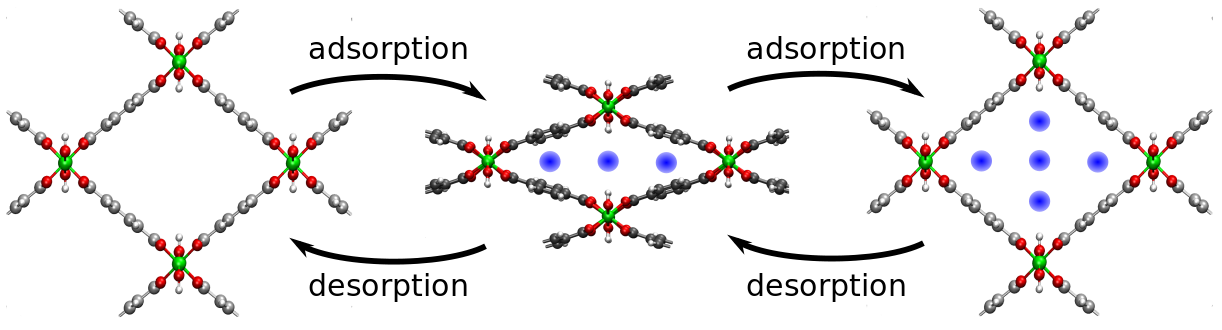
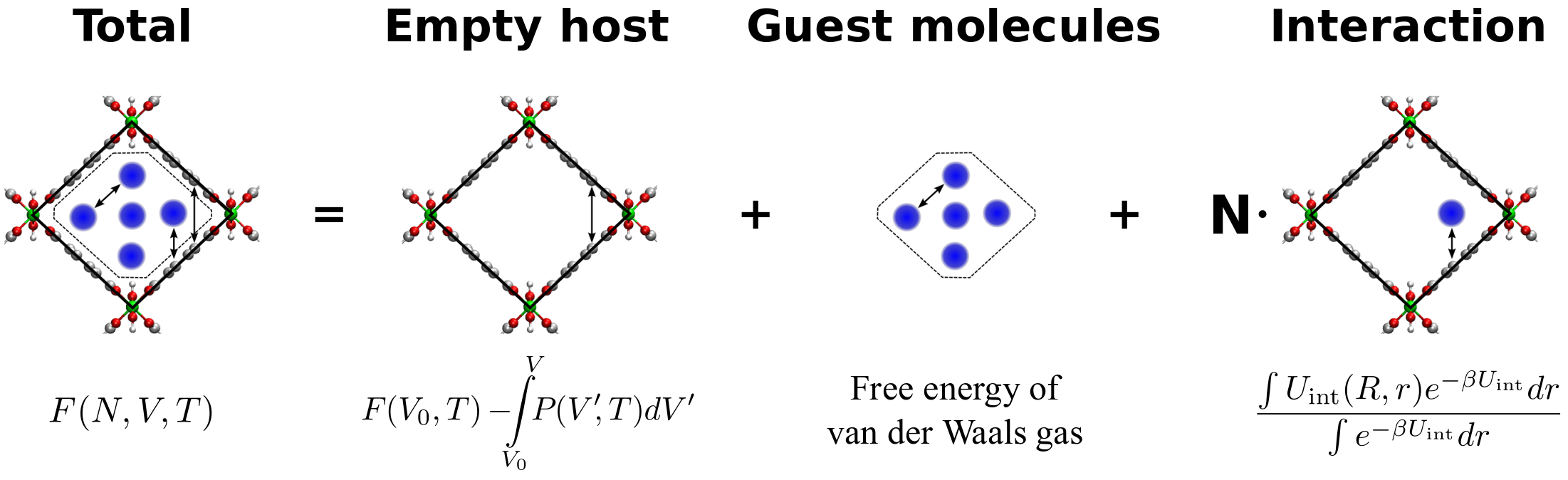
Recently, a thermodynamic model has been proposed at the Center for Molecular Modeling (CMM) to describe breathing behavior under influence of mechanical pressure as well as adsorption of guest molecules. This model starts from an analytical expression of the free energy as function of the unit cell volume and possible the number of guest molecules. To compute the amplitude of the framework transformations, an explicit Legendre transform is performed to transform the Helmholtz free energy of a fixed volume ensemble to the free energy in a fixed pressure ensemble, which also results in the equilibrium volume at the given pressure. A crucial part of the model is the free energy profile of the empty framework as function of the unit cell volume. Several enhanced techniques were developed at the CMM to determine such free energy profiles based on molecular dynamics and Monte Carlo simulations. Up to now, these free energy profiles are computed at fixed temperature and the influence of the temperature is not clear.
Goal
In this thesis, the student will first investigate the temperature dependence by computing the free energy (and hence entropy) at various temperatures using advanced molecular dynamics and/or monte carlo methods. Next, the student will try to capture the temperature dependence by proposing an analytical temperature-dependent variant of the thermodynamical model and extracting the extra mathematical parameters from the simulations. To validate the methodology, the student could first start with simple systems such as harmonic oscillators. In a later stage, the free energy of a well-known flexible framework MIL-53 will be computed. The outcome of the thesis topic will contribute to the thermodynamic model describing the breathing behavior of metal-organic frameworks.
This topic is on the cross road between statistical physics, thermodynamics and molecular simulation. On the one hand you'll need to understand the limitations of standard simulation techniques and perform enhanced simulation techniques to obtain the free energy. Understanding this type of simulations requires good understanding of statistical physics. The topic will deepen your knowledge about simulation techniques and computational physics. If needed, the required programming skills will be transferred during the thesis.
Aspects
Physics: Development of advanced thermodynamic and statistical physics models
Engineering: Application of thermodynamic models to investigate the temperature dependence of several important properties of nanoporous materials.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]KeywordsThermodynamics, Free energy calculations, entropy, temperature, molecular simulations, Nanoporous materials


