Stability of carbenium ions in a complex zeolitic environment
Stability of carbenium ions in a complex zeolitic environment
Promotor(en): V. Van Speybroeck, K. De Wispelaere /18NANO03 / Nanoporous materialsMany conversions in acid zeolites occur through carbenium ion intermediates. Alkene oligomerization or cracking and methanol-to-hydrocarbon (MTH) conversion are typical examples of complex conversions in which carbenium ions play a crucial role. The stability and reactivity of such cations, however, is dictated by the nature of the hydrocarbon, the nature of the active site (ranging from Brønsted to Lewis acid sites), the degree of confinement within the zeolite pore and the operating conditions. How each of these factors determines the stability of carbenium ions is not well understood. Recently, we discovered that operating conditions have a major influence on the nature and stability of alkene intermediates in H-ZSM-5.1 A proper estimate of the stability of carbenium ion intermediates is a necessary condition for predicting accurate reaction kinetics of complex catalytic . Due to the high reactivity of the reaction intermediates at operating conditions, it is hard to acquire this information from an expemental viewpoint. Therefore, ab initio methods are an indispensable tool for unravelling the governing intermediates and reaction mechanism.
Nowadays, many molecular modeling studies represent zeolites as idealized materials containing isolated Brønsted acid sites. In reality, however, zeolites are more complex and contain extraframework aluminums (EFAls) with Lewis acidic properties, vicinal Brønsted acid sites and active sites at the external surface. Each of these sites may have a distinct role, which can be elucidated by advanced molecular simulations. Recently, zeolite models have been proposed that represent the structure of the external surface of a zeolite crystal (Figure 1).2
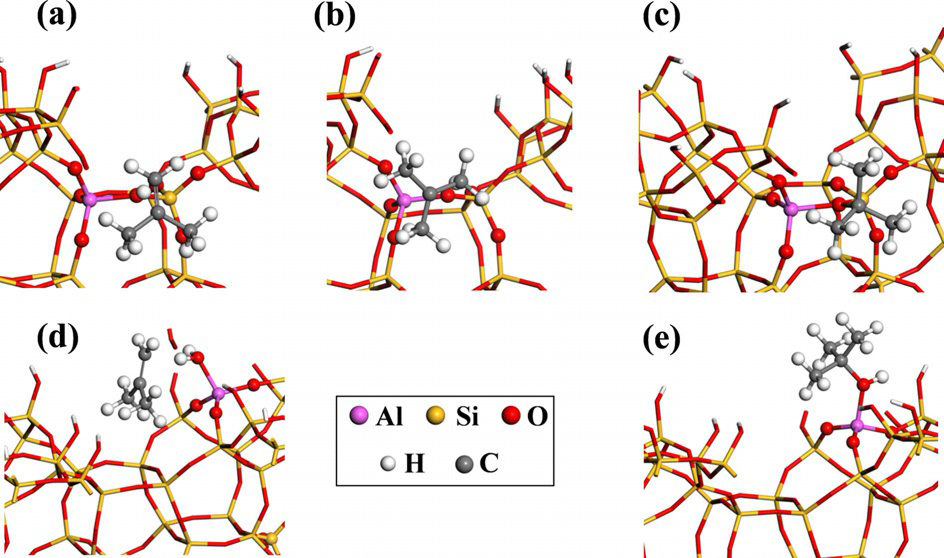
Figure 1. Isobutene adsorption intermediates at the external surface of zeolite beta in the open micropore in the vicinity of a bridging OH group.Objectives
In the first phase of this project, the student is expected to construct advanced zeolite models, representing various types of active sites and the external surface of the zeolite. This will require the application of state-of-the-art static and molecular dynamics (MD) simulations. Such MD simulations will be necessary to determine the stability and optimal position of extraframework species in the zeolite pores. After a detailed characterization of the active sites, the second phase will consist of assessing the stability of a broad range of hydrocarbon intermediates at the various active sites. To mimick realistic operating conditions, again MD simulations will be applied.
This project requires the application of several advanced molecular simulation techniques, which rely on first principle static and molecular dynamics simulations. The Center for Molecular Modeling has built up vast expertise in these advanced simulation techniques and collaborates on the subject with leading experimental and theoretical partners. The student will be actively coached to get acquainted with the plethora of techniques needed to tackle the proposed problem. It is the intention to involve the student actively in the work discussions with our collaborators. The CMM has access to sufficient computational resources to execute this research project. The proposed topic is challenging and requires technical skills, creativity and chemical insight.
Aspects
Chemical aspect: Structure determination, unraveling stability of reaction intermediates
Engineering aspect: Application to the catalytic properties of nanoporous materials
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, carbenium ionsRecommended coursesMoleculaire modellering van industriële processen, Simulations and Modeling for the NanoscaleReferences
[1] P. Cnudde et al., J. Catal., 345 (2017) 53-69.
[2] J. Rey et al., ChemCatChem, 9 (2017) 2176-2185.

