Screening co-solvents for improving the conversion of of biomass in complex reaction environments.
Screening co-solvents for improving the conversion of of biomass in complex reaction environments.
Promotor(en): V. Van Speybroeck /20CHEM02 / Chemistry & BiochemistryProbleemstelling:
Due to a growing world population and an increasing demand for resources, society is obliged to look for sustainable alternatives to replace the current fossil-based economy. Biomass, consisting of (hemi)cellulose and lignin, is an interesting option to replace petroleum-based feedstocks. provide replacements for petroleum-based compounds. Up-to-date mainly (hemi)cellulose derived products have been investigated and found their use in every-day society. In contrast, lignin, which is a biopolymer rich in aromatic moieties, has found only limited application possibilities due to its complex nature. Lignin is currently produced as a byproduct of paper- and pulp industry which is mostly burnt afterwards for energy recovery. However, recently the lignin-first concept was proposed which first selectively extracts the lignin from the biomass leaving the (hemi)cellulose fraction untouched. The diverse aromatic polymer matrix of lignin can then be converted to a limited number of monomers.1 These monomers can be either used directly for the production of fine-chemicals but mostly need to be converted into building blocks such as catechol and guaiacol. Recently Bomon et al. discovered that hot-pressurized water, in combination with homogeneous Brønsted acid catalysts, provides an efficient way to selectively defunctionalize these monomeric arenes.2 Water (in the subcritical state) plays a crucial role within this process both as a solvent and as a reactant. Understanding the role of water and possible synergetic effects arising from the use of a co-solvent, such as methanol, is hence of huge importance to optimize process conditions and increase product yield. However, a sustainable alternative for homogeneous Brønsted acids are zeolites which offer many advantages: not only can they be trivially separated from the reaction mixture via simple filtration, but they can also improve the reaction selectivity thanks to the enclosed environment of the pores. A clear understanding of the differences in the reactivity of lignin-derived phenolic compounds between the pure liquid phase and the constrained environment of zeolites is then of fundamental importance to drive the research towards more efficient dealkylation processes.
Doelstelling:
Within this master thesis you will try to understand the role of co-solvents in water in the defunctionalization (O-demethylation and C-dealkylation) of lignin-derived monomers in hot-pressurized water. Due to the subcritical conditions at which these reactions are typically performed (T >250 °C, P > 50 bar), state-of-the-art molecular dynamics simulations will be used to investigate the role of the co-solvent at operating conditions. Because the influence of co-solvents on the reactivity is rather unexplored, accurate insights will first be obtained by purely considering the liquid phase at operating conditions. In a next step, you will also investigate the solvent mixture within a zeolite framework, to elucidate how the narrow pore dimensions and the hydrophobic environment affect its behaviour and how it interacts with the material active sites.
In a second stage you will look into the effect of these co-solvents on the intrinsic reactivity patterns of the lignin-derived monomers at operating conditions. Furthermore, you will also investigate the effect of different degrees of mixing of the co-solvent with pure water both in the pure liquid phase and in zeolites. The overall results of the simulations will result in a proper characterization of the solvent-zeolite system and an improved understanding of the mechanism for lignin valorization.
The Center for Molecular Modeling (CMM) has a large experience in both the study of homogeneous-catalyzed and zeolite-catalyzed reactions with advanced molecular simulations. You will be coached to become acquainted with the software and techniques required to study the systems of interest. The computational power necessary for the calculations will be ensured by CMM’s access to the main national supercomputing infrastructures. The proposed research topic is extremely relevant in the contemporary chemistry research. You will be actively involved in the ongoing collaboration with the experimental group of of Prof. Bert Maes (University of Antwerp) and Prof. Bert Sels (KU Leuven.
(1) Van Den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S. F.; Renders, T.; De Meester, B.; Huijgen, W. J. J.; Dehaen, W.; Courtin, C. M.; et al. Reductive Lignocellulose Fractionation into Soluble Lignin-Derived Phenolic Monomers and Dimers and Processable Carbohydrate Pulps. Energy Environ. Sci. 2015, 8 (6), 1748–1763.
(2) Bomon, J.; Van Den Broeck, E.; Bal, M.; Liao, Y.; Sergeyev, S.; Van Speybroeck, V.; Sels, B. F.; Maes, B. U. Brønsted Acid Catalyzed Tandem Defunctionalization of Biorenewable Ferulic Acid and Derivates into Bio-Catechol. Angew. Chemie Int. Ed. 2019.
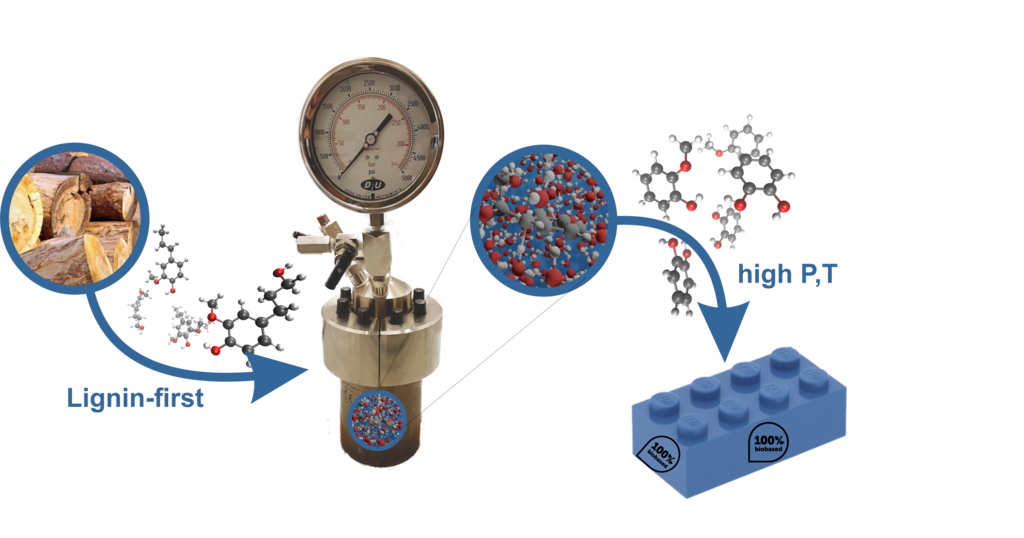
Figure 1. Schematic representation of the lignin-first process and a subsequent conversion towards bio-renewable building blocks in hot pressurized water.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsModelling, Nanoporous materials, catalysis, hot pressurized solvent
