Reaction routes for C-C formation in the CO2-to-hydrocarbons process
Reaction routes for C-C formation in the CO2-to-hydrocarbons process
Promotor(en): V. Van Speybroeck /20NANO03 / Nanoporous materialsProbleemstelling:
Atmospheric CO2 emissions need to be reduced drastically to mitigate the impact of global warming. Currently, the development of technologies to capture this greenhouse gas and directly transform it into valuable chemicals receives a lot of interest. Promising results in the CO2 reductive valorization are obtained with a combination of conventional metallic catalysts and acid zeolites. [1-3] The metallic catalyst is used to convert CO2 into CO, which is in turn converted into hydrocarbons through classical Fischer-Tropsch synthesis. The acid zeolite increases the light olefin and aromatics yield by transformation of these hydrocarbons through cracking, aromatization, isomerization reactions, … Alternatively, the metallic catalyst can convert CO2 into methanol, which is further processed in the zeolite framework through the methanol-to-hydrocarbons (MTH) chemistry. To optimize these bifunctional catalysts, fundamental insight into the governing reaction mechanisms is essential.
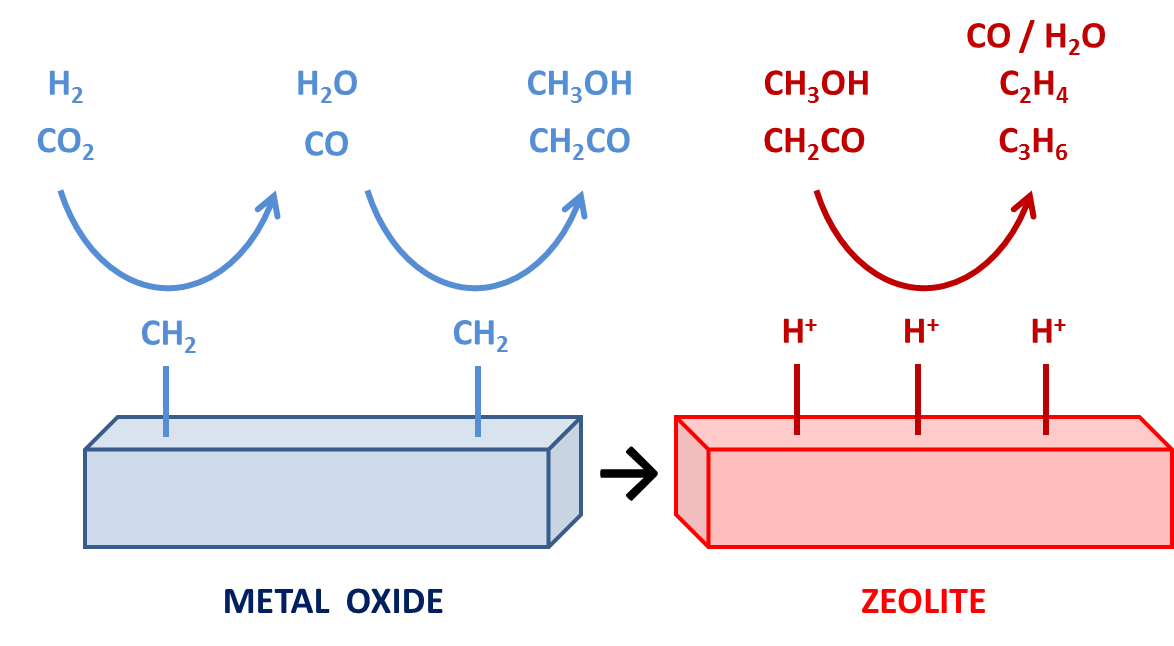
Figure 1. Illustration of the oxide-zeolite bifunctional catalyst to convert CO2 into light olefins. [4]
By selecting a proper zeolite catalyst, a higher light olefin yield or a higher aromatics yield can be achieved. One of the main challenges is to tune the product distribution towards the desired products such as propene. This is complicated by the many competitive reactions (cracking, hydrogenation, alkylation …) taking place simultaneously and by the presence of CO, CO2, H2O and H2 in the zeolite pores which can affect reactions between hydrocarbons. For example, carbonylation reactions have similarities with C-C coupling reactions in the methanol-to-hydrocarbons process. Ketenes and surface formates are proposed as important intermediates to control the selectivity of this process. [4-5] However, these are very reactive, short-lived species and their role in the formation of hydrocarbons is not yet unravelled. Nevertheless, a full understanding of the reaction mechanism is essential to progress towards more efficient CO2 conversion catalysts.
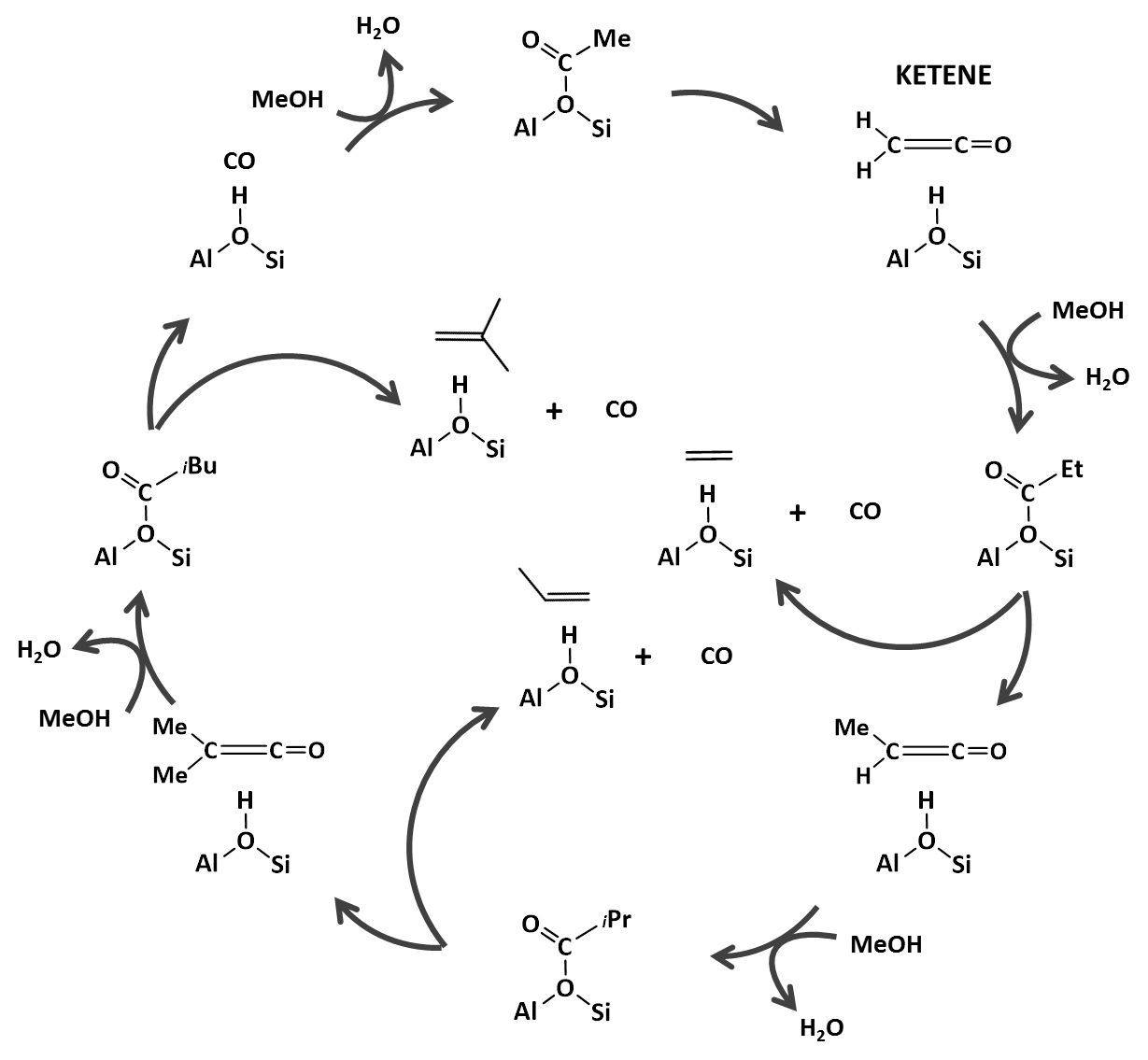
Figure 2. Proposed ketene-based mechanism for the formation of light olefins during the zeolite catalyzed MTH process. [4]
Doelstelling:
The goal of this master thesis is to investigate how conventional MTH reactions (both in the initiation phase and steady state operation) are affected by the presence of CO, CO2, H2 and H2O. First, you will evaluate the stability of various reactive intermediates (ketene, surface formate …) which can be formed in a hydrogenative environment. Afterwards, key pathways involving these intermediates in the C-C coupling and hydrocarbon transformation mechanism will be simulated to assess the importance of the presence of CO in the reaction mixture. To discriminate between competitive pathways and to take into account the complex molecular environment, first-principle molecular dynamics simulations at operating conditions will be performed. As the role of the zeolite is crucial for fine-tuning of the product distribution towards light olefins or aromatics, you will perform this study in zeolites with different pore topologies. This analysis will yield insight in the role of CO and H2 on hydrocarbon transformations in zeolites and will assist in the improvement of light olefin selectivity of CO2 conversion processes.
The Center for Molecular Modeling has ample experience in modeling of zeolite catalysis with advanced simulation techniques. The proposed topic is situated in a very active research field. The student will be actively coached to get acquainted with the multitude of available techniques to tackle the proposed problem. The CMM has access to large scale computational infrastructure for studying this complex system. The student will be actively involved in the running collaboration with prof. Jorge Gascon (KAUST) on this topic.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, ZeolitesReferences
[1] A. Ramirez et al., ACS Catal. 8 (2018) 9174-9182.
[2] J. Wei et al., Nat. Comm. 8 (2017) 15174.
[3] P. Gao et al., Nat. Chem. 9 (2017) 1019-1024.
[4] A.D. Chowdhury and J. Gascon, Angew. Chem. Int. Ed. 57 (2018) 14982-14985.
[5] A.D. Chowdhury et al., Angew. Chem. Int. Ed. 128 (2016) 16072-16077.
