Reaction path analysis in methanol to olefins conversion
Reaction path analysis in methanol to olefins conversion
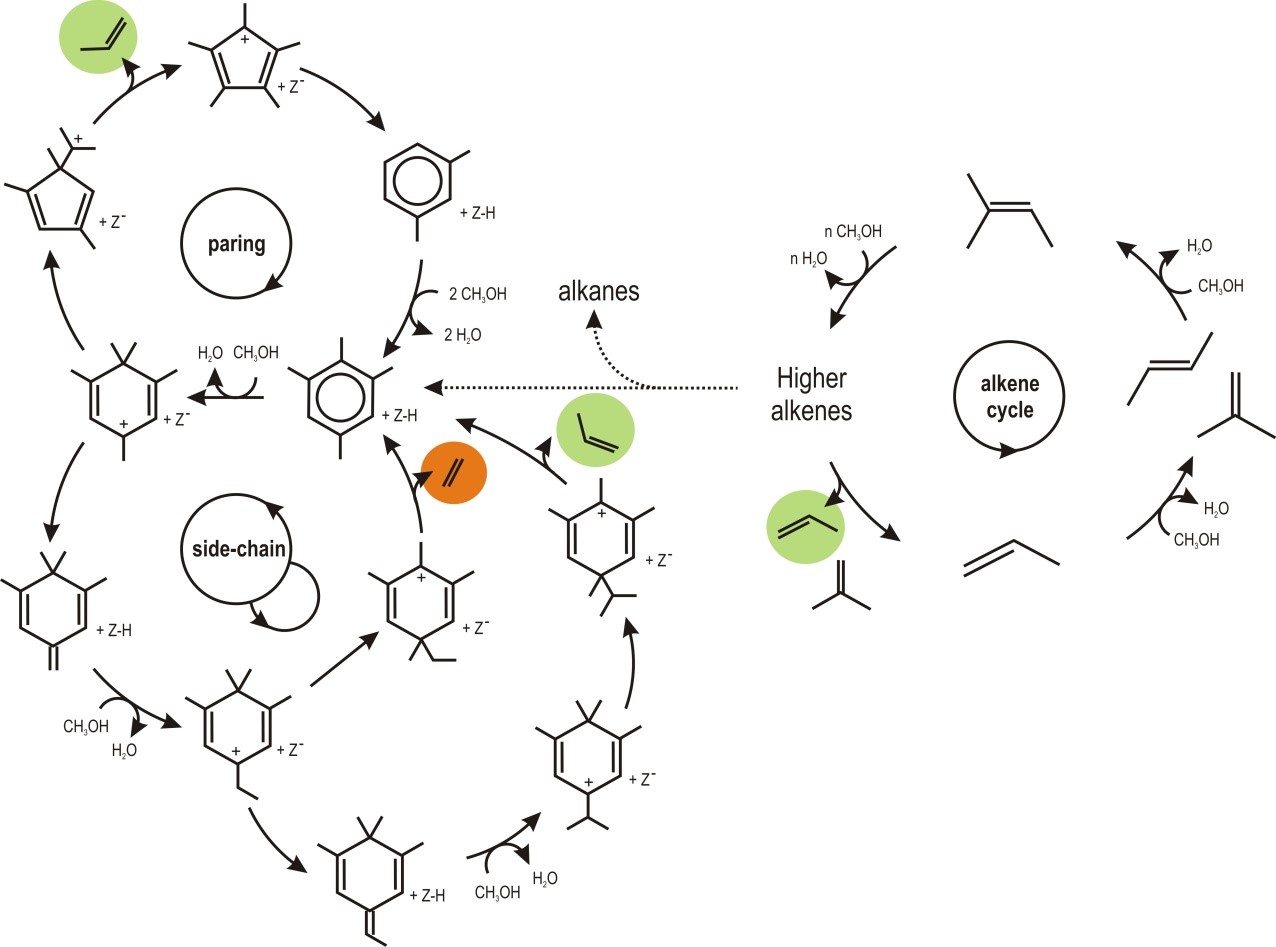
Promotor(en): J.W. Thybaut, V. Van Speybroeck /18NANO05 / Nanoporous materialsRapidly declining fossil reserves have urged the exploration of alternative feeds and corresponding routes towards similar chemicals and fuels. Methanol to olefins conversion is such a proven technology which is, at present, operated on the industrial scale. Methanol is contacted with an acid catalyst that provokes carbon-carbon bond coupling and formation of heavier hydrocarbons, c.q., alkenes. Apart from the acidity, the pore topology, i.e., the framework type, of the catalyst used is one of the determining factors for the activity and, maybe even more importantly, the selectivity. Indeed, catalyst performance is deemed to be governed by so-called hydrocarbon pool species which undergo methylation, isomerization and dealkylation reactions. The rather bulky nature of these species explains the specific reactions they can be subject to and the corresponding preferential product formation while that of others is sterically hindered. An alternative mechanism, i.e., alkene homologation, typically prevails when the hydrocarbon pool mechanism experiences severe steric constraints or when the catalyst exhibits a mild acid strength.
Both the Catalytic Reaction Engineering group (CaRE) within the Laboratory for Chemical Technology and the Centre for Molecular Modelling (CMM) have a strong track record in methanol to olefins conversion. While CaRE has focused on microkinetic model development, i.e., expressing reaction rates in terms of elementary steps, in a top-down manner, the CMM has adopted a bottom-up approach starting from an ab initio based analysis of the reaction mechanism. In the present project, a synergy will be pursued by feeding the microkinetic model with ab initio based insight, c.q., parameter values. Depending on the interests of the student, this thesis may focus more on chemical intuitions or on governing physical interactions at the atomic scale.
Objectives
The assumptions as presently used in the microkinetic methanol to olefins conversion simulations [1,2] will be evaluated in view of the ab initio acquired insight in the reaction mechanism.[3] To this end results of high level static DFT calculations complemented with insights from molecular dynamics simulations will be compared to parameter estimates in the microkinetic model. The combined modeling approach should allow identifying relevant features that may need to be incorporated into the microkinetic model. It is expected to lead to extended reaction pathways involving parallel routes. A so-called ‘reaction pathway analysis’ will be used as a tool to distinguish the most relevant pathways from rather unimportant ones. The impact of the operating conditions such as temperature and feed composition on the reaction pathway analysis results should, of course, be kept in mind. In particular the influence of the zeolite topology will be assessed by comparing the ZSM-5 and ZSM-23 materials. Indeed, the reaction species are strongly influenced by topological aspects through weakly bonding interactions, such as hydrogen bridges and van der Waals forces.
The CaRE and CMM have ample experience and access to sufficient computational resources to execute this research project. The proposed topic is challenging and requires technical skills, creativity and chemical insight to tackle this problem from a molecular and microkinetic modeling viewpoint.Aspects
Physics aspects: insights in atomic-scale interactions and use of quantum mechanical programs
Engineering aspects: application to molecular reactions in nanoporous materials
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Chemical Engineering [EMCHEM], Master of Science in Sustainable Materials Engineering [EMMAEN]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) NANO, MATERIALSKeywordsZeolites, Heterogeneous Catalysis, Chemical kinetics, Computational applications, microkinetic modelingReferences
[1] Kumar, P.; Thybaut, J. W.; Svelle, S.; Olsbye, U.; Marin, G. B. Ind. Eng. Chem. Res. 2013, 52, 1491-1507.
[2] Kumar, P.; Thybaut, J. W.; Teketel, S.; Svelle, S.; Beato, P.; Olsbye, U.; Marin, G. B. Catal. Today 2013, 215, 224-232.
[3] Hemelsoet, K.; Van der Mynsbrugge, J.; De Wispelaere, K.; Waroquier, M.; Van Speybroeck, V. ChemPhysChem 2013, 14, 1526-1545.

