Phase transformations in binary Fe-N alloys
Phase transformations in binary Fe-N alloys
Promotor(en): S. Cottenier, S. Claessens /16MAT04 / Solid-state physicsJust like carbon, nitrogen is one of the most effective solid solution strenghteners in steel. Especially in austenitic stainless steels it is commonly used as cheap alloying element. Moreover, nitrogen at the same time enhances the corrosion resistance, particularly in Cl-containing environments. To be fully able to exploit the advantages of nitrogen, it is therefore important to understand how it reacts with iron. Although the Fe-N phase diagram is rather well-known and looks quite similar to the Fe-C phase diagram, certain aspects remain uncertain today. In particular, the kinetics of transformations during cooling are not well described. This includes the formation and kinetics of metastable phases (‘martensite-like’ or ‘bainite-like’) but also the stability of the Fe4N phase. This phase can be compared to the classic Fe3C (cementite) in the Fe-C diagram. However, apart from the thermodynamic similarities, Fe4N and Fe3C have a dissimilar crystallography (see figure below). How these differences influence nucleation behavior is very valuable knowledge, as it can help to discern which known characteristics from the Fe-C system are transferable to the Fe-N system.
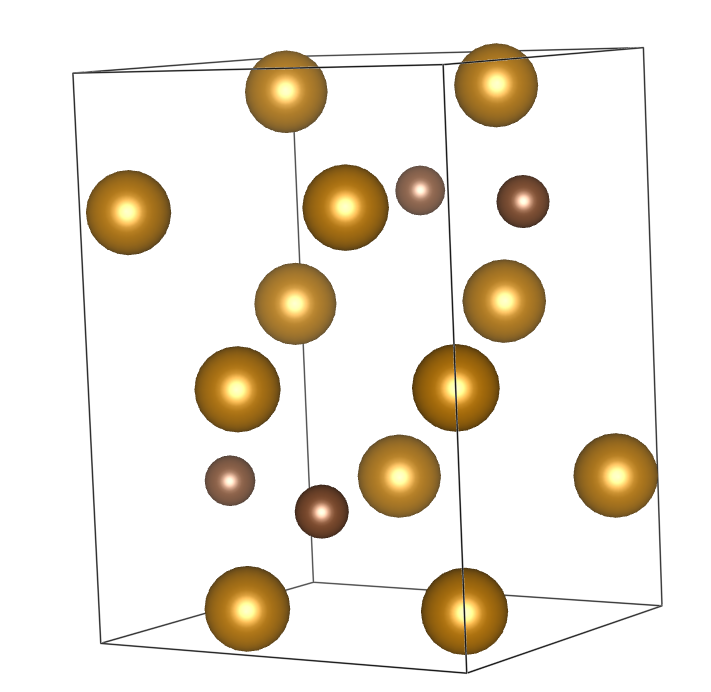
Left: Fe4N; Right: Fe3CGoal
How you wish to tackle the research depends on your specific interests and background. One of the strong points of this subject is that it ties in with current research at the Center for Molecular Modeling (CMM) and OCAS NV. As such, the thesis student will have the chance to work closely with academic and industrial experts on a highly relevant topic, helping to improve the understanding of the transformation behavior in the Fe-N binary system, which is essential for future product developments.
The Fe4N material is the point of departure for this thesis project. The more specific research question is “What influences the precipitation rate of Fe4N in the Fe-N matrix?” The precipitation rate is influenced by the thermodynamics of both Fe with N in solid solution and the Fe4N phase. Apart from that, the kinetics of the phase transformation to Fe4N and the interface between the Fe matrix and the Fe4N precipitate are decisive as well. A few detailed problems that can be addressed computationally are:
- What is the difference between diffusion of N in the Fe bulk and via grain boundaries?
- How do ternary elements impact the diffusion of N?
- How do ternary solutes impact the relative stabilities of Fe4N and Fe with N in solution?
- Are there any metastable intermediary phases facilitating Fe4N precipitation?
- Crystallographically, what interface energies determine the precipitate morphology?
Besides the computational part, there is also valuable input to be gained from experiments. The impact of cooling rates and aging times on microstructure evolution, morphology changes and hardness evolution can be determined by dilatometer testing on binary Fe-N alloys with various N-contents.
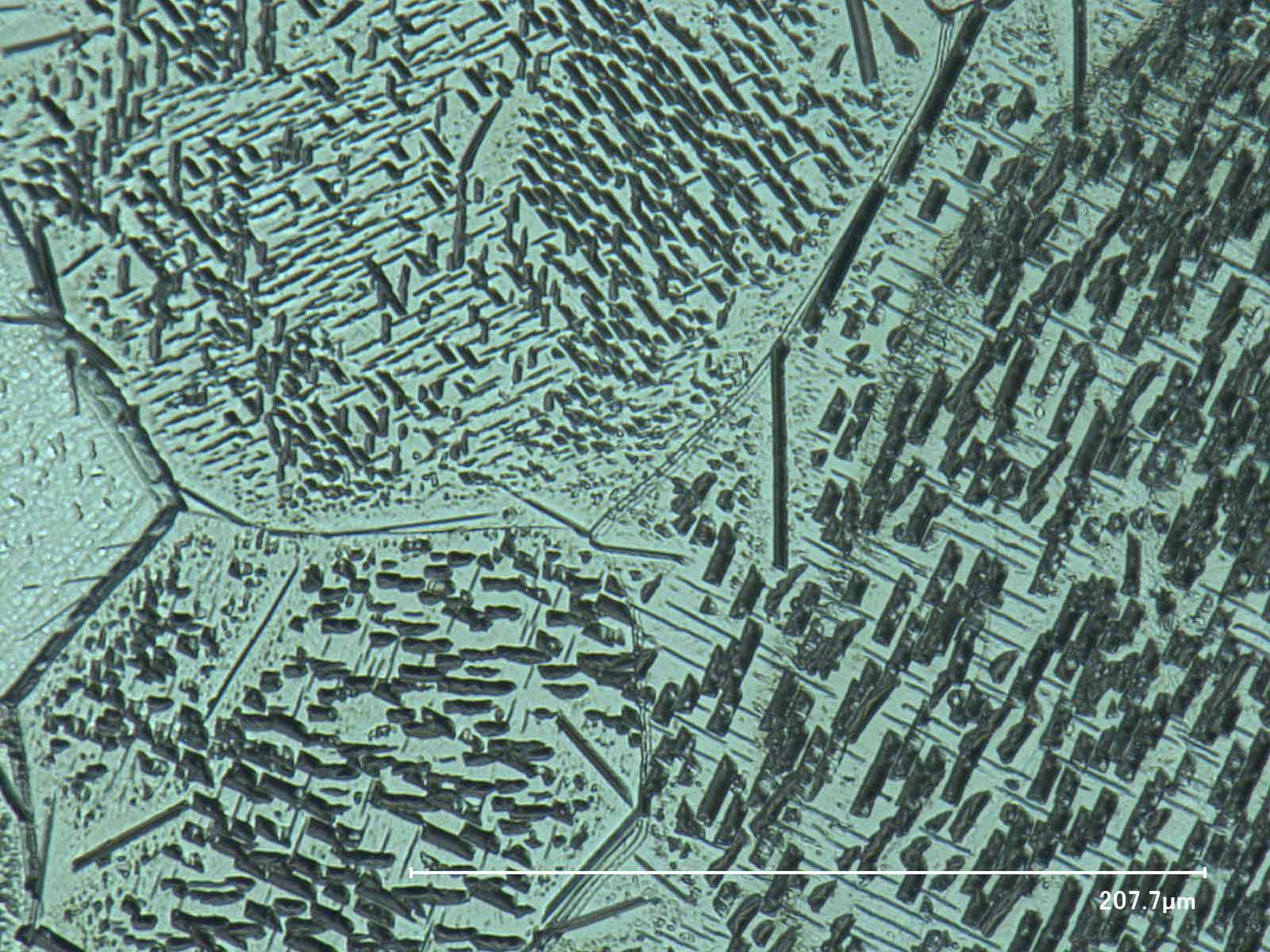

Left: Nitrogen is precipitated out of the Fe matrix in the form of Fe4N (slower cooling rate). Right: Nitrogen is in solid solution in the Fe matrix (faster cooling rate).Physics & Engineering aspects
Physics aspect: use of quantum physical methods to characterize materials
Engineering aspect: application to the properties of metals
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Sustainable Materials Engineering [EMMAEN], Master of Science in Physics and Astronomy [CMFYST]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) MODELING, MATERIALS, NANO



