Operando vibrational spectroscopy to unravel the nature of intermediates in nanoporous materials
Operando vibrational spectroscopy to unravel the nature of intermediates in nanoporous materials
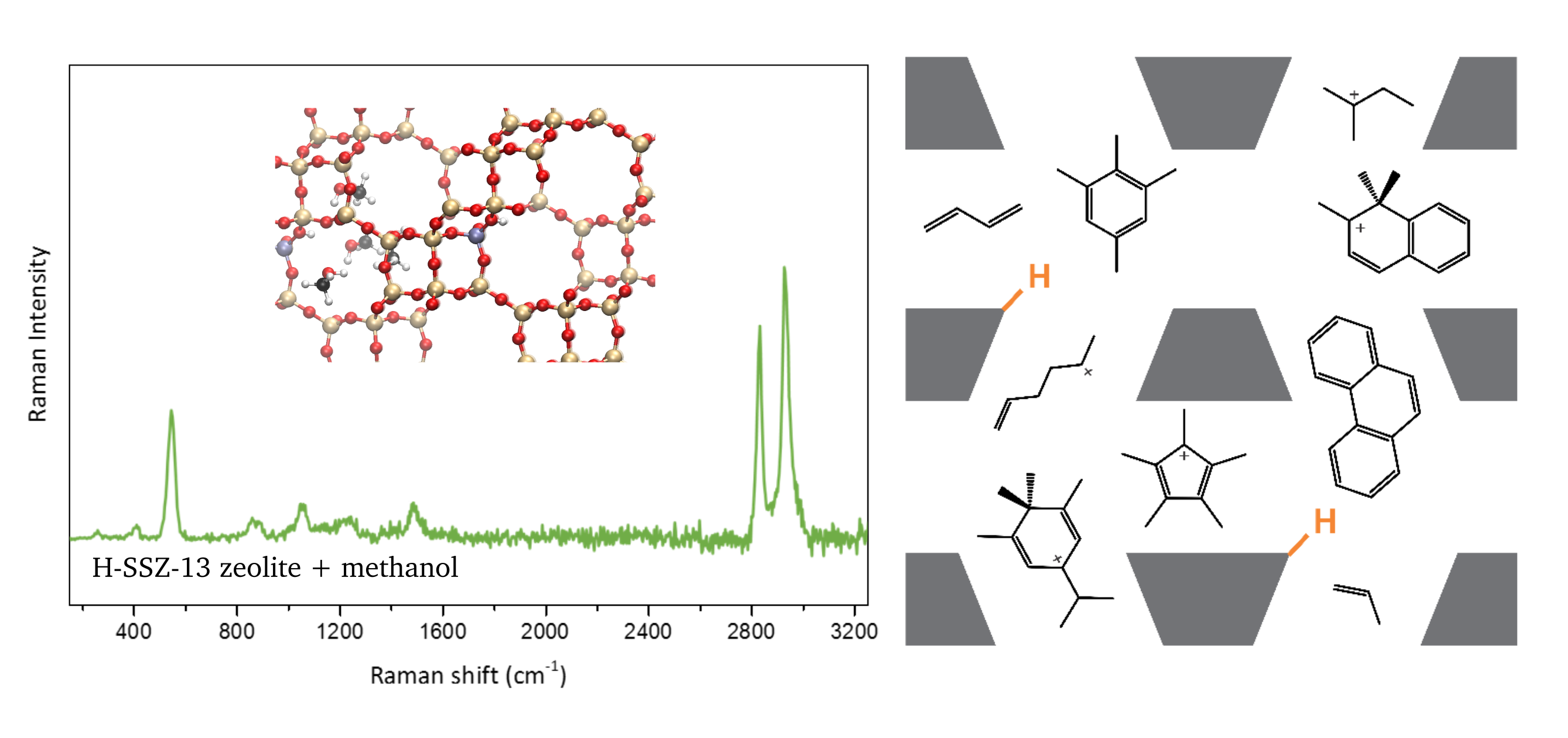
Promotor(en): V. Van Speybroeck /18SPEC06 / Nanoporous materials, SpectroscopyZeolites are an important class of porous, crystalline materials. Their suitability for applications in gas adsorption and catalysis turned them into the workhorses of present-day petrochemical industry. A particularly interesting industrial process involving zeolites is the conversion from methanol to light olefins, i.e. the methanol-to-olefin (MTO) process. It has attracted a lot of interest the last decades as it is believed to be a linkage between coal or natural gas chemical industry and modern petrochemical industry. This process is extremely complex and to date not completely understood as it involves numerous molecules, i.e. so-called intermediates, appearing along the reaction process [1]. Insight in the mechanism can be obtained by following these molecules in the zeolite via operando spectroscopic techniques.
A wide range of spectroscopic techniques exist as characterization tools in zeolite materials science. Recent advances made operando spectroscopic measurements possible, leading to a wealth of information while the system is at work. Vibrational spectroscopy, among which infrared (IR) spectroscopy is most frequently used, is especially well-suited to follow molecules in catalytic reactions involving zeolites [2]. It provides structural information on the zeolite, which is important to understand the changes in the host framework during catalytic processes. Moreover, it is the ideal technique to get insight in the reaction mechanisms as each molecule has specific fingerprint vibrations. Nowadays, a lot of experimental research making use of operando vibrational spectroscopic techniques has been performed to shed light upon, for example, the MTO process. However, the complicated mechanism involving plenty of different molecules results in vibrational spectra which are hard to interpret with only experimental input.Computational vibrational spectroscopy can greatly facilitate the interpretation of the obtained experimental spectra and can even be used to predict the evolution of signals in terms of varying composition or functionality of the material [3]. The field has seen a major leap forward the last decade due to a continuous expansion of computer power on one side and the development of advanced theoretical models on the other side. Given these advances, it has become possible to simulate very challenging systems such as light olefins in zeolites. As a consequence, computational spectroscopy has evolved into an indispensable tool to unravel the observed spectra and to connect the obtained insights with plausible reaction mechanisms for the MTO process.
Goal
The goal of this thesis is to obtain a deeper understanding of the reaction mechanism governing the MTO process via computational IR spectroscopy. This requires an accurate prediction of the vibrations in the system, which urges the use of methods whereby the Schrödinger equation needs to be solved. Your objective is to get insight in the lattice vibrations in the zeolite and the effect of molecules such as light olefins on these vibrations. This will enable an evaluation of several proposed mechanisms in the methanol conversion.
To complete the main goal, you will employ two fundamentally different approaches. A first approach is the static one where a normal mode analysis will be performed around a minimum of the potential energy surface (PES). This method makes use of the harmonic approximation, but allows for a full quantum mechanical treatment of the problem. In contrast, the vibrational spectra can also be generated by means of molecular dynamics (MD) simulations, in which various configurations of phase space are sampled on a quantum mechanically based PES. The different configurations are obtained by solving Newton’s equations of motion at each time step. Following this approach, anharmonicities are inherently taken into account. Another advantage of MD over static simulations, which is of special interest in this thesis, is the possibility to perform simulations at operating conditions by imposing temperature and pressure via simulations in the NPT ensemble.
This research consists of different steps leading toward the main goal. In a first step, you will investigate the lattice vibrations of several empty frameworks with the MFI topology by generating the corresponding IR spectrum using both static and dynamic simulations. You will evaluate the performance of both methods by comparing your results with experimental data. Once a clear picture of the lattice vibrations of the empty zeolite is obtained, you will investigate the effect of different molecules present in the pores on the lattice vibrations. The research can possibly be extended to zeolites with different topologies. Finally, this will allow you to elucidate the plausible mechanisms for the MTO process and to suggest other possibilities for this industrially relevant process.
For this thesis proposal you can rely on experimental reference data provided by the Beale group from University College London with whom the CMM has initiated a collaboration. Furthermore, you will have to opportunity to be involved in work discussions with this experimental partner.
Aspects
Engineering: The student will apply advanced molecular simulations to understand the complex MTO process, which is of high industrial importance.
Physics: The student will use several techniques and interpret their results, which requires a fundamental understanding of quantum mechanics, solid state physics and atomic and molecular physics.
- Study programmeMaster of Science in Engineering Physics [EMPHYS]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) NANO, MODELINGKeywordsIR spectroscopy, MTO process, Molecular modelingReferences
[1] K. Hemelsoet, J. Van der Mynsbrugge, K. De Wispelaere, M. Waroquier, and V. Van Speybroeck, Unraveling the reaction mechanisms governing methanol-to-olefins catalysis by theory and experiment, ChemPhysChem 14, p. 1526-1545 (2013)
[2] S. Bordiga, C. Lamberti, F. Bonino, A. Travert, and F. Thibault-Starzyk, Probing zeolites by vibrational spectroscopies, Chem. Soc. Rev. 44, p. 7262-7341 (2015)
[3] V. Van Speybroeck, K. Hemelsoet, L. Joos, M. Waroquier, R. G. Bell, C. R. A. Catlow, Advances in theory and their application within the field of zeolite chemistry, Chem. Soc. Rev. 44, p. 7044-7111 (2015)

