Modeling and design of new acid-cleavable linkers
Modeling and design of new acid-cleavable linkers
Promotor(en): R. Hoogenboom, V. Van Speybroeck /MM_14_CHEM_02 / Chemistry & BiochemistryPoly(2-oxazoline)s (POX) are biocompatible polyamides that are readily synthesized and whose properties can easily be tuned. These polymers are synthesized via cationic ring-opening polymerization of various monomers, yielding polymers with distinct properties. Moreover, different properties can be obtained by chemical functionalization of the side chain of these polymers after polymerization. Because of their versatility and biocompatibility, poly(2-oxazoline)s are candidate drug-delivery systems and possible alternatives for poly(ethylene glycol) (PEG), the gold standard in the field.
The introduction of an acid-cleavable linker between the (anti-cancer) drug and the poly(2-oxazoline)s is a prerequisite for therapeutic applications. More specifically, this linker should be stable at pH 7.4 so that the drug remains attached to the polymer in the blood stream and after cellular uptake the drug should be rapidly released to become active triggered by the lower pH of ~5 during the endosomal uptake. This project will focus on the design of new cleavable linker strategies that are suitable for use with POX will be developed and optimized. Molecular modeling will be used to provide an initial screening and evaluate the prospect of several linker systems, initially based on carbamate and acetal linkers with various substituents.
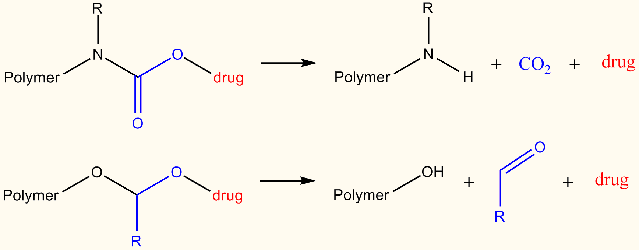
More specificially, DFT calculations will be performed on relatively small model systems describing the linker and its closest molecular environment. Bond dissociation energies will be calculated for vulnerable bond(s) in the linker, providing a quick way to establish its strength as a function of various substituents. This initial in-silico screening of substituents will limit the number of wet-lab experiments that need to be conducted. In addition, also complete simulations will be conducted of the various dissociation reactions of the linkers yielding direct mechanistic insight, possibly leading to identification of chemical modifications to target for promoting or reducing cleavage. Moreover, comparison of the resulting calculated thermodynamic and kinetic data for various substituted linkers will give an enhanced view of their relative stability, especially when the dissociation reaction involves several discrete reaction steps. This information could furthermore be applied to alter reaction conditions favoring either kinetic or thermodynamic control.
- Study programmeMaster of Science in Chemistry [CMCHEM], Master of Science in Biochemistry and Biotechnology [CMBIBI]

