Mechanistic study on the zeolite-catalyzed formation of aromatics with advanced molecular simulations
Mechanistic study on the zeolite-catalyzed formation of aromatics with advanced molecular simulations
Promotor(en): V. Van Speybroeck /17NANO02 / Nanoporous materialsAromatics are ubiquitous in hydrocarbon chemistry. During alcohol conversion into hydrocarbons they can play an active or deactivating role in the hydrocarbon pool mechanism and during the catalytic cracking of alkanes and alkenes they deactivate the catalyst. Studies on the aromatic formation in zeolitic materials are rather limited and suggest two possible mechanisms, depicted in Figure 1. One possible mechanism is based on the oligomerization and cyclization of olefins as crucial steps. [1] Another possible explanation for the formation of aromatic intermediates might be the cycloaddition reactions between conjugated dienes and alkenes, known as Diels-Alder type reactions. These type of reactions have already been reported in zeolites containing dehydrogenation metallic sites and acidic sites [2] and for furan conversion into aromatics and olefins over Brønsted and Lewis acidic zeolites in the area of biomass upgrading.[3] Recently, also the role of oxygen-containing intermediates like aldehydes have been suggested during hydrogen-transfer reactions which are typically coupled with aromatics formation.[4]
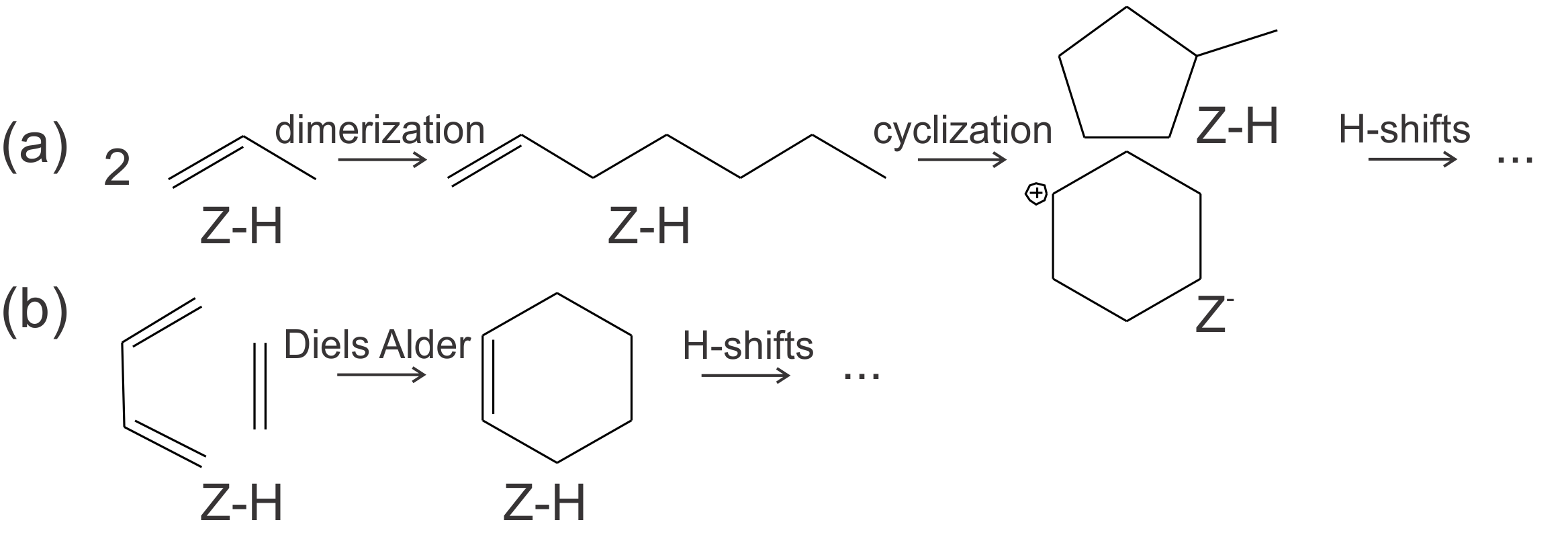
Figure 1. Schematic representation of the possible reaction mechanisms for the formation of aromatics, namely oligomerization and cyclization (a) and Diels-Alder type reactions (b).Aromatic intermediates are of particular interest in H-SAPO-34, used for the methanol-to-olefin (MTO) process, since the topology of this catalyst permits the formation of aromatics, which play an active role in the methanol conversion. The interest in the conversion of methanol over zeolitic materials to hydrocarbons (MTH) or olefins has grown the last decades, since it is one of the most prominent technologies to bypass crude oil in light olefin production. Intensive research to elucidate the mechanism governing the methanol conversion has shown that the zeolitic material is aided by an organic co-catalyst that is occluded in the framework. Reaction cycles based on hydrocarbons as co-catalysts are known as the hydrocarbon pool (HP) mechanism. Although the production of olefins with aromatics as co-catalysts is generally accepted, there is still no consensus on the origin of these aromatics formed during the early stages.
Goal
The objective of this master thesis is to propose reaction mechanisms for the formation of aromatics in H-SAPO-34 and check their feasibility at realistic MTO reaction conditions. To this end first principle chemical kinetics simulations will be performed on various elementary reactions leading to aromatic species. A significant part of the project will also be devoted to studying the role of aldehydes in the formation of aromatics. The calculated kinetics can then serve as input for a micro-kinetic model, which allows assessing the influence of operating conditions (temperature, pressure) on the aromatics formation. In a second step, some crucial reaction steps will be selected to assess the influence of the zeolite topology on the mechanism of aromatics formation. It is well known that some zeolites can efficiently suppress the formation of aromatics [5] and more systematic insights into this effect are key to a further optimization of the MTH catalyst.
This project requires the application of several advanced molecular simulation techniques, which rely on first principle static and molecular dynamics simulations. The Center for Molecular Modeling has built up vast expertise in these advanced simulation techniques and collaborates on the subject with leading experimental and theoretical partners. The student will be actively coached to get acquainted with the plethora of techniques needed to tackle the proposed problem. It is the intention to involve the student actively in the work discussions with our collaborators. The CMM has access to sufficient computational resources to execute this research project. The proposed topic is challenging and requires technical skills, creativity and chemical insight.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Chemical kinetics, Computational applicationsReferences
[1] M. Vandichel, D. Lesthaeghe, J. V. der Mynsbrugge, M. Waroquier, and V. Van Speybroeck, “Assembly of cyclic hydrocarbons from ethene and propene in acid zeolite catalysis to produce active catalytic sites for MTO conversion,” J. Catal., vol. 271, no. 1, pp. 67–78, Apr. 2010.
[2] K. D. Hungenberg, J. H. Kagon, and B. E. Langner, “Evidence for a Diels-Alder reaction between dienes and olefins in the dehydrocyclodimerization of butene-1 on tellurium-loaded zeolites,” J. Catal., vol. 68, no. 1, pp. 200–202, Mar. 1981.
[3 N. Nikbin, S. Feng, S. Caratzoulas, and D. G. Vlachos, “p-Xylene Formation by Dehydrative Aromatization of a Diels–Alder Product in Lewis and Brønsted Acidic Zeolites,” J. Phys. Chem. C, vol. 118, no. 42, pp. 24415–24424, Oct. 2014.
[4] S. Müller, Y. Liu, F.M. Kirchberger, M. Tonigold, M. Sanchez-Sanchez, J.A. Lercher, "Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons", J. Am. Chem. Soc., 138 (2016) 15994-16003
[5] S. Teketel, U. Olsbye, K.P. Lillerud, P. Beato, S. Svelle, Selectivity control through fundamental mechanistic insight in the conversion of methanol to hydrocarbons over zeolites, Microporous Mesoporous Mater., 136 (2010) 33-41.

