A joint experimental/theoretical approach to synthesize iridium covalent organic frameworks as heterogeneous catalysts for aerobic alcohol oxidation
A joint experimental/theoretical approach to synthesize iridium covalent organic frameworks as heterogeneous catalysts for aerobic alcohol oxidation
Promotor(en): V. Van Speybroeck, P. Van der Voort /17NANO14 / Chemistry & BiochemistryIn recent years, nanoporous, ordered materials have attracted a lot of attention in diverse fields such as gas adsorption, catalysis of chemical reactions, and nanosensing applications. As these materials possess both large internal pores and a long-range ordered structure, they are ideally suited to use as host materials for guest adsorption. In recent years, two large classes of materials have emerged: metal-organic frameworks (MOFs), and, more recently, covalent organic frameworks (COFs) [1]. COFs, being comprised solely of strong covalent bonds (see Figure 1), possess an outstanding chemical, thermal, and mechanical stability. Moreover, given the possibility to tune the geometry and size of their pores, COFs have a huge potential to be used as support materials for highly functional heterogeneous catalysis.
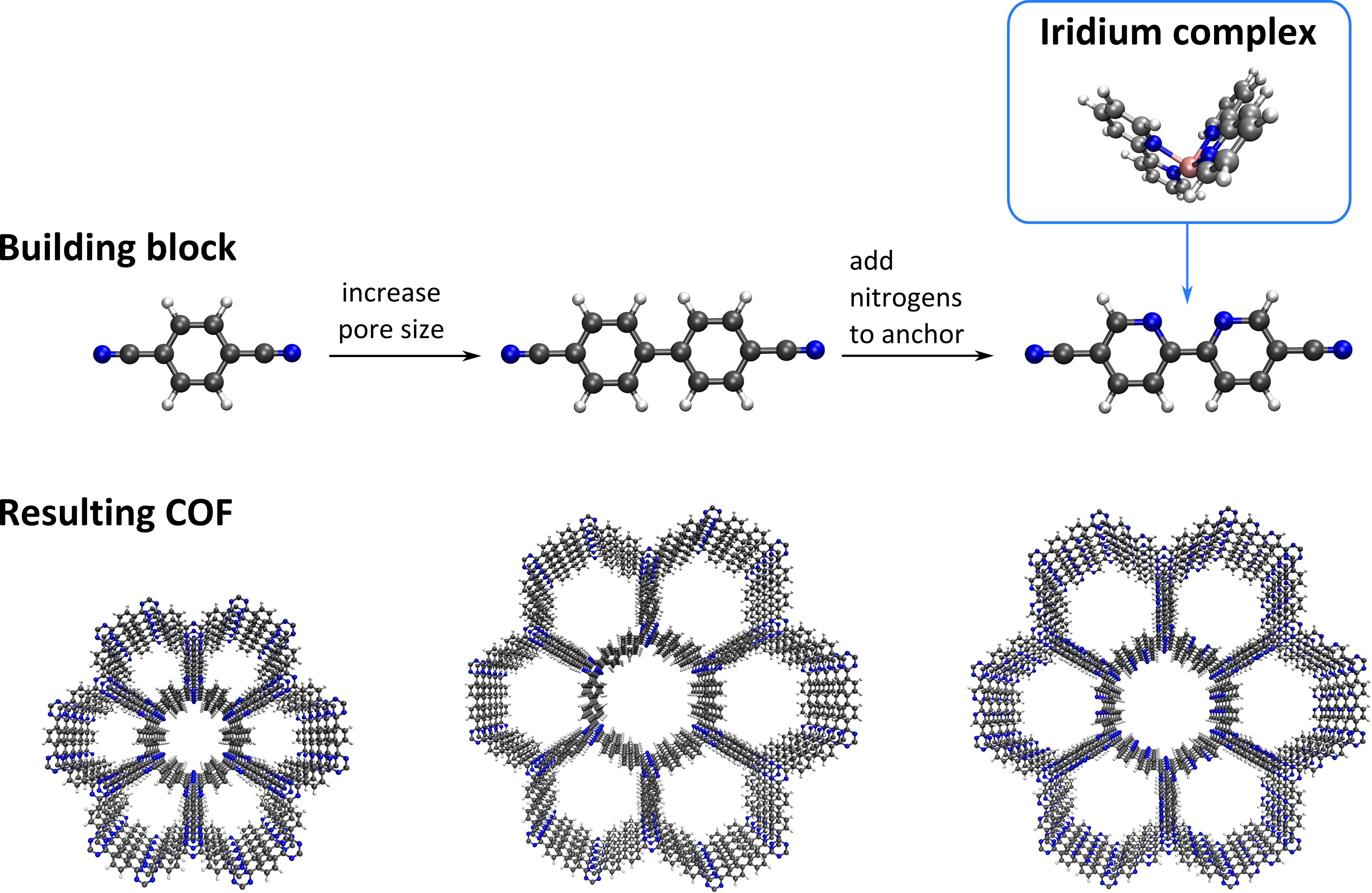
To overcome the non-recycling ability of homogeneous catalysts, immobilizing them onto a solid support offers many advantages. Within this context, a number of homogeneous complexes have been anchored onto various supports such as titanium oxide, organic polymers, and ion-exchange resins, with varying success. To ensure that the catalytically active complexes do not leach, they should be anchored to the support material with strong and stable bonds. Moreover, the pore size of the support should be sufficiently large to allow for the reactants to also reach the active complexes located deeper inside the support. These criteria can be easily met by choosing appropriate COF building blocks, for instance using a bipyridine moiety (see Figure 1). The 2,2’-bipyridine functional group is the most widely employed chelating ligand for forming metal complexes in coordination chemistry, since it can form metal complexes in which the metal is bound to the two nitrogen atoms to make it a bidentate-secured stable complex. Here, we are especially focusing on anchoring iridium complexes onto bipyridine ligands, as shown in Figure 1.
Goal
During this project, you will get the opportunity to synthesize bipyridine-based COFs that will be used afterwards as a catalytic support material for the coordinative anchoring of iridium(III) complexes at the Centre for Ordered Materials, Organometallics and Catalysis (COMOC). From an environmental point of view, it is of paramount importance to develop stable catalysts that use O2 as a clean and cheap oxidant to realize a so-called “green oxidation” process. Within this context, the application of iridium(III) complexes is far less explored in comparison to other transition metals. However, iridium complexes have already been shown to be promising catalysts under severe thermal or basic conditions [2], and are hence ideally suited to be complexated into stable COFs.
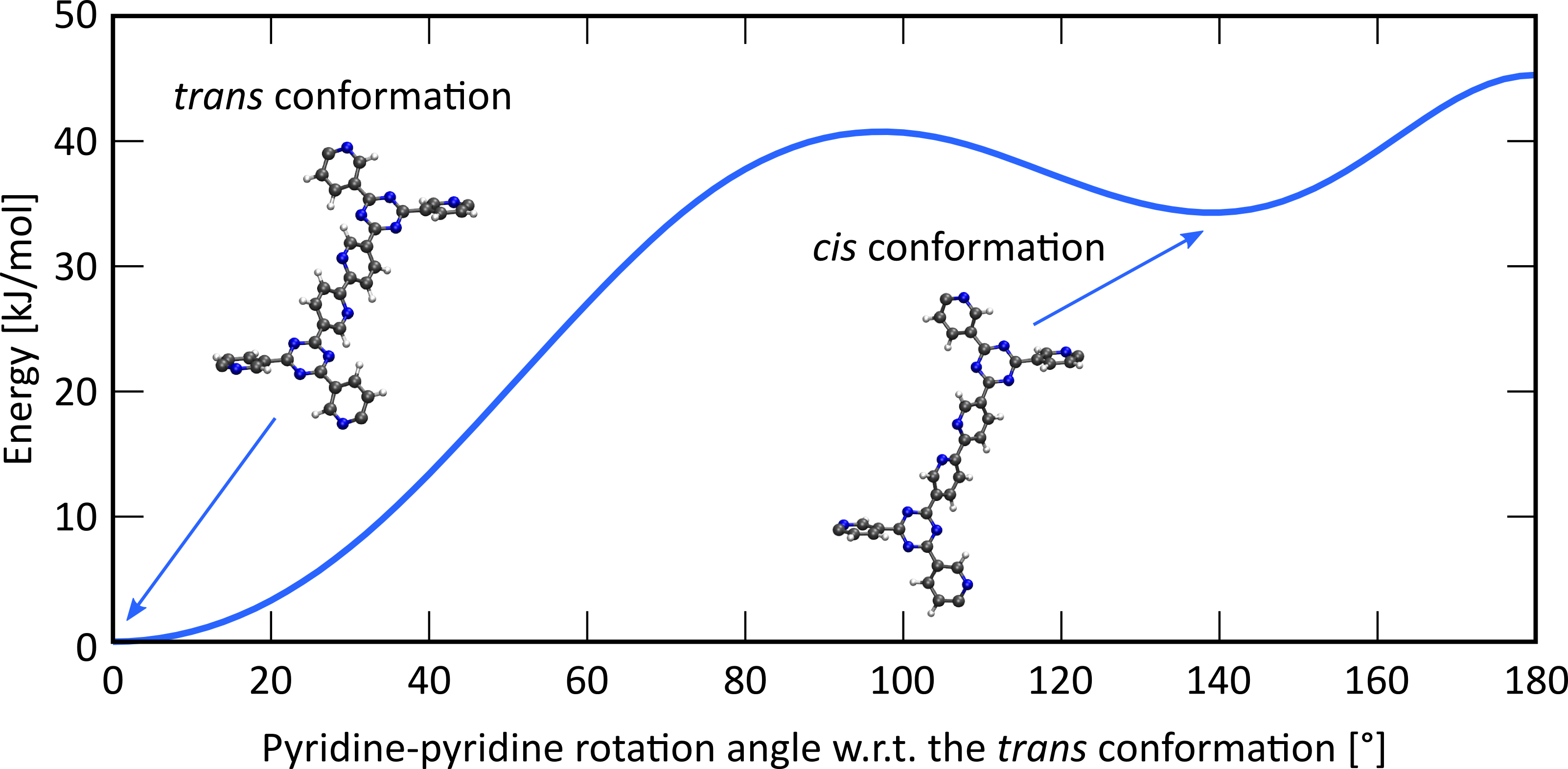
To help understand the complexation of this iridium complex on the bipyridine ligands, you will quantum mechanically investigate the interactions between the bipyridine ligand and the metal complex at the Center for Molecular Modeling (CMM), using density functional theory (DFT) to yield complementary insight to the aforementioned synthesis. To this end, a representative cluster model will be cut from the periodic structure, ensuring that the cluster size is still large enough to accurately model the periodic structure. While experimental studies reveal that these complexes preferentially anchor on the two nitrogens of the bipyridine ligand (in the cis conformation), also complexation on one nitrogen and one carbon has been reported [3], which occurs when one of the two pyridine ligands rotates over 180° (trans conformation, see Figure 2). As an extension, you will also study how several Brønsted acid complexes interact with both the bipyridine ligand and the terminating CN moieties of the building blocks. These acid complexes should, ideally, coordinate to the –CN terminating groups of the building blocks, since this hugely facilitates the condensation of these buildings blocks into the final COF material. However, experimental results indicate that these acid complexes preferentially coordinate to the bipyridine moiety. Complementary theoretical insight might reveal which interactions are dominant in the process. Identifying these interactions and constructing linkers or identifying acid complexes such that these interactions are reduced will result in a reproducible protocol which can be adopted to successfully synthesize COFs on which iridium complexes can be anchored and subsequently be employed as heterogeneous catalysts for aerobic alcohol oxidation.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM], Master of Science in Chemistry [CMCHEM]KeywordsCovalent organic frameworks, Porous materials, Complexation, Molecular simulation, First principles, SynthesisReferences
[1] A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, "Porous, crystalliny, covalent organic frameworks," Science, vol. 310, no. 5751, pp. 1166-1170, 2005.
[2] S. Abednatanzi, P. G. Derakhshandeh, A. Abbasi, P. Van Der Voort and K. Leus, "Direct synthesis of an iridium(III) bipyridine metal-organic framework as a heterogeneous catalyst for aerobic alcohol oxidation," ChemCatChem, vol. 8, no. 23, pp. 3672-3679, 2016.
[3] M. S. Lowry, W. R. Hudson, R. A. Pascal Jr. and S. Bernhard, "Accelerated luminophore discovery through combinatorial synthesis," J. Am. Chem. Soc., vol. 126, no. 43, pp. 14129-14135, 2004.


