Hydrogen storage and transport in methane clathrates: the kinetics of hydrogen adsorption and diffusion
Hydrogen storage and transport in methane clathrates: the kinetics of hydrogen adsorption and diffusion
Promotor(en): V. Van Speybroeck /25656 / Nanoporous materialsBackground and problem
Hydrogen and fuel cells have been named as sustainable energy solutions for the 21st century, providing a way to reduce carbon dioxide emissions and transition to clean energy sources. However, the widespread application of hydrogen is limited by technological problems that arise from energy inefficient hydrogen storage and transportation technologies. A promising solution to mitigate this problem is the use of hydrogen storage in ice-like clathrate hydrates, although some hurdles still have to be overcome to use this technology in future practical applications. Challenging research topics in this field include the formation of clathrate structures at ambient conditions and understanding the process of adsorption and diffusion in these materials. Adsorption of hydrogen in nanoporous materials or chemisorption in metal hydrides has also been explored, but suffers from irreversibility in the storage and release of hydrogen, a limited storage capacity, etc.
Clathrate hydrates are structures similar to ice, but made up out of cages large enough to host small guest molecules (Figure 1). In nature methane clathrate hydrates can be found in deep-sea sediments. The first hydrogen clathrates were synthesized only two decades ago [1], mainly due to the slow kinetics, extremely high pressures (about 200 bar), and low temperatures (about 250 K) involved in this process. Therefore, such a process is not economically viable on an industrial scale.
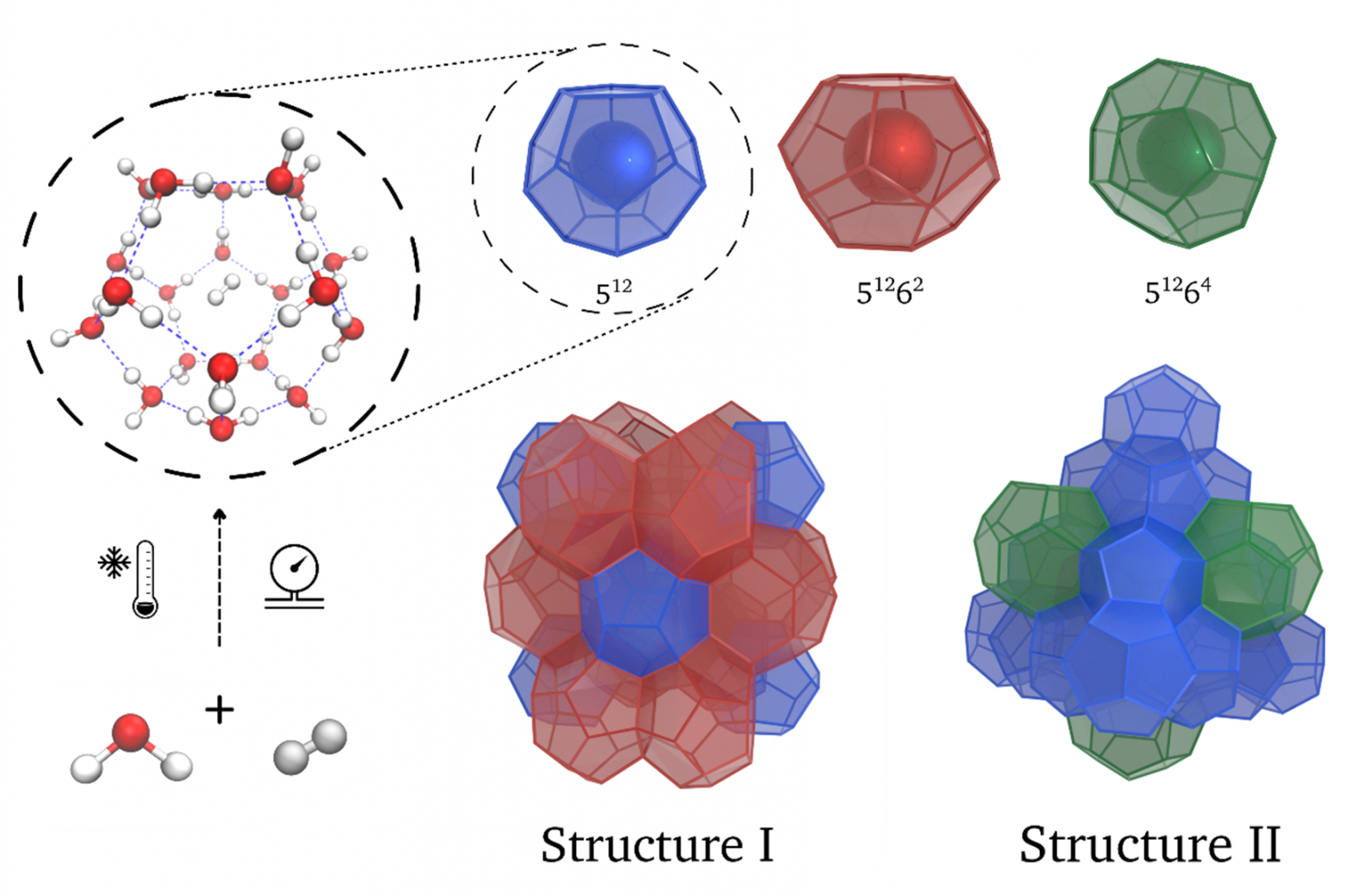
Figure 1. Hydrogen clathrates: ice-like structures formed in cryogenic and high-pressure conditions with water molecules organized in ordered cages with x pentagonal (5x) and y hexagonal (6y) faces. For commercial applications, their synthesis needs improving.
Other clathrates, such as methane hydrates show less severe formation conditions, which may be further improved by employing inner-cage promotors (e.g. THF, alkanes) [2], surface stabilizers (e.g. surfactants) [3] and nanoconfinement in porous materials [4]. The latter has shown to be particularly promising, with several nanoporous materials displaying considerable potential for clathrate storage.
As methane clathrates are easier to form than hydrogen clathrates, a particularly interesting concept is the formation of methane clathrates in mild conditions under nanoconfinement followed by a flushing with H2 to replace the CH4 in the clathrate template matrix (Figure 2). This process is already employed in geological methane clathrate deposits, where CO2 is flushed both for CH4 harvesting and CO2 sequestration.
Currently, this process is however ill-understood and a fundamental understanding of both the adsorption of H2 in the clathrate structure as well as the kinetics of H2 diffusion in the presence of other guest species such as CH4 is mandatory to further lift this concept to a technologically viable solution. This thesis will be performed in close collaboration with a network of experimental partners in Flanders, who are synthesizing the materials and studying various energetic and kinetic aspects related to hydrogen adsorption and diffusion through the clathrate matrix.
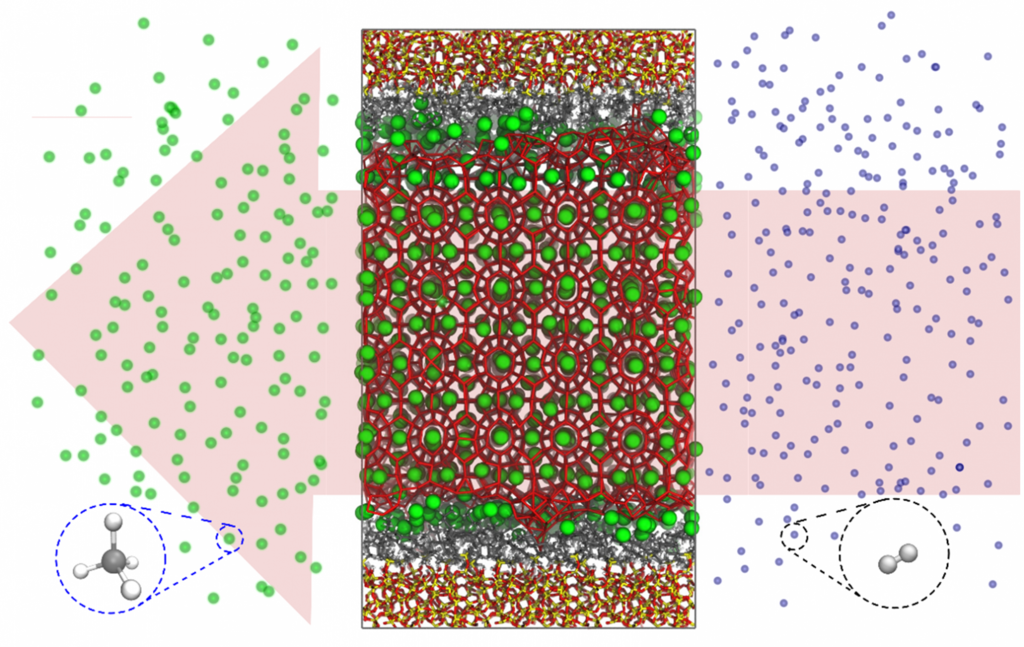
Figure 2. Gas displacement in confined methane clathrates: occupation of a confined methane (green sphere) clathrate network by H2 molecules (blue spheres) under a pressure gradient represented by the arrow (high-pressure zone at the bottom of the arrow; low-pressure zone at the top of the arrow).
Goal
Within this thesis, we will start from an available model for methane clathrates that already proved its merits (Figure 2), where a clathrate structure is confined in a larger porous material. Using state-of-the-art regular and enhanced-sampling molecular dynamics (MD) simulations, the student will evaluate the energetics of hydrogen adsorption in the clathrate structure, gradually substituting CH4 by H2, mimicking what occurs in the clathrate network in operando conditions. Furthermore, also multiple H2 molecules occupying the clathrate cages will be considered. Besides an energetic and kinetic assessment of the process of hydrogen flushing, the thermodynamic/kinetic model will also allow to assess the gravimetric and volumetric capacities of the clathrates and compare them with currently available technologies such as liquid organic carriers and other more standard nanoporous frameworks.
To describe the clathrate systems, an effective force field potential will be used to allow for the simulation of larger systems over longer timescales in contrast to ab initio methods that solve the Schrödinger equation directly. The time evolution of the system is obtained by performing MD simulations in which Hamilton’s equations of motion are integrated throughout time. This enables us to track the displacement of the gas molecules and their possible effect on the hydrogen bonded network, including local hydrogen bond breaking and network self-healing. Due to the low mass of the protons, nuclear quantum effects (NQEs) such as zero-point energy can however yield important contributions to the description of water and hydrogen, and will potentially have to be taken into account. To study the diffusion of guest molecules in the clathrates, which can involve considerable energy barriers, enhanced sampling techniques can be used to facilitate the transitions, since some transitions only occur rarely in regular MD simulations. Finally, interesting adsorption configurations resulting from the analysis of the hydrogen flushing process can also be investigated using first-principles quantum mechanical simulations on smaller clathrate unit cells to further improve the description of the adsorption energies.
By applying this methodology, the goal is to propose a reasonable mechanism for gas displacement and complement experimental findings recently obtained from NMR experiments by our experimental collaborators. Incidentally, this proposal is under the scope of a larger initiative, the ARCLATH moonshot project. This investigation effort engages academic research and industrial partners for developing new hydrogen storage, transport and delivery systems.
During the thesis year, the student will be actively coached in the various aspects of the simulations and will be actively involved in the network of experimental partners to cross validate his/her results with the experimental data.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]Keywordshydrogen storage, kinetics of H2 diffusion and adsorption of H2 in porous materialsReferences
[1] Y. A. Dyadin, É. G. Larionov, E. Y. Aladko, A. Y. Manakov, F. V. Zhurko, T. V. Mikina, V. Y. Komarov and E. V. Grachev, J. Struct. Chem., 1999, 40, 790–795.
[2] I. N. Tsimpanogiannis, I. G. Economou and A. K. Stubos, J. Chem. Eng. Data, 2020, 65, 1289–1299.
[3] H. P. Veluswamy, J. Y. Chen and P. Linga, Chem. Eng. Sci., 2015, 126, 488–499.
[4] C. Cuadrado-Collados, J. Farrando-Pérez, M. Martínez-Escandell, L. A. Ramírez-Montoya, J. A. Menéndez, A. Arenillas, M. A. Montes-Morán and J. Silvestre-Albero, Chem. Eng. J., 2020, 402, 126276.
