How flushing can save the hydrogen economy: Understanding diffusion processes in porous ice crystals
How flushing can save the hydrogen economy: Understanding diffusion processes in porous ice crystals
Promotor(en): V. Van Speybroeck /28143 / Nanoporous materialsBackground and problem
To reduce our dependency on fossil fuels and the resulting carbon dioxide emissions, the use of hydrogen as an energy vector could further catalyze the transition towards more sustainable energy sources. In particular the production of hydrogen at moments when renewable energy production exceeds the demand can help to realize the full potential of these renewable energy sources. However, due to its low density and high flammability, the safe and efficient storage and transport of hydrogen remains a challenge.1
A very promising way of storing hydrogen gas is by means of so-called clathrates.1,2 Clathrates are solid water crystals formed through hydrogen bonds, similar to ice, but which unlike ice contain pores in which small molecules are encapsulated. Since these molecules are located in different cages, the transport and storage of these flammable gases inside clathrates is much safer, even under the high pressures necessary to make this procedure economically feasible. Depending on the specific guest molecules, typically methane or hydrogen gas, different clathrate structures can be formed as shown in Figure 1. While especially hydrogen clathrates are interesting for hydrogen storage, pure hydrogen clathrates are only formed at very low temperatures and high pressures. As a result, different synthesis methods are investigated to ease the formation conditions, which include nanoconfinement in porous materials or the use of promotor molecules such as tetrahydrofuran. Recently, together with collaborators from the KU Leuven, we turned our attention to the opportunity to first form methane clathrates instead, as these can be grown under mild conditions, and then replace methane with hydrogen through a so-called flushing process, as shown in Figure 2. While experimental results are promising, it remains unclear how this flushing process proceeds as well as how it is affected by incorporating the clathrate in a hydrophobic porous material or by including promotor molecules.
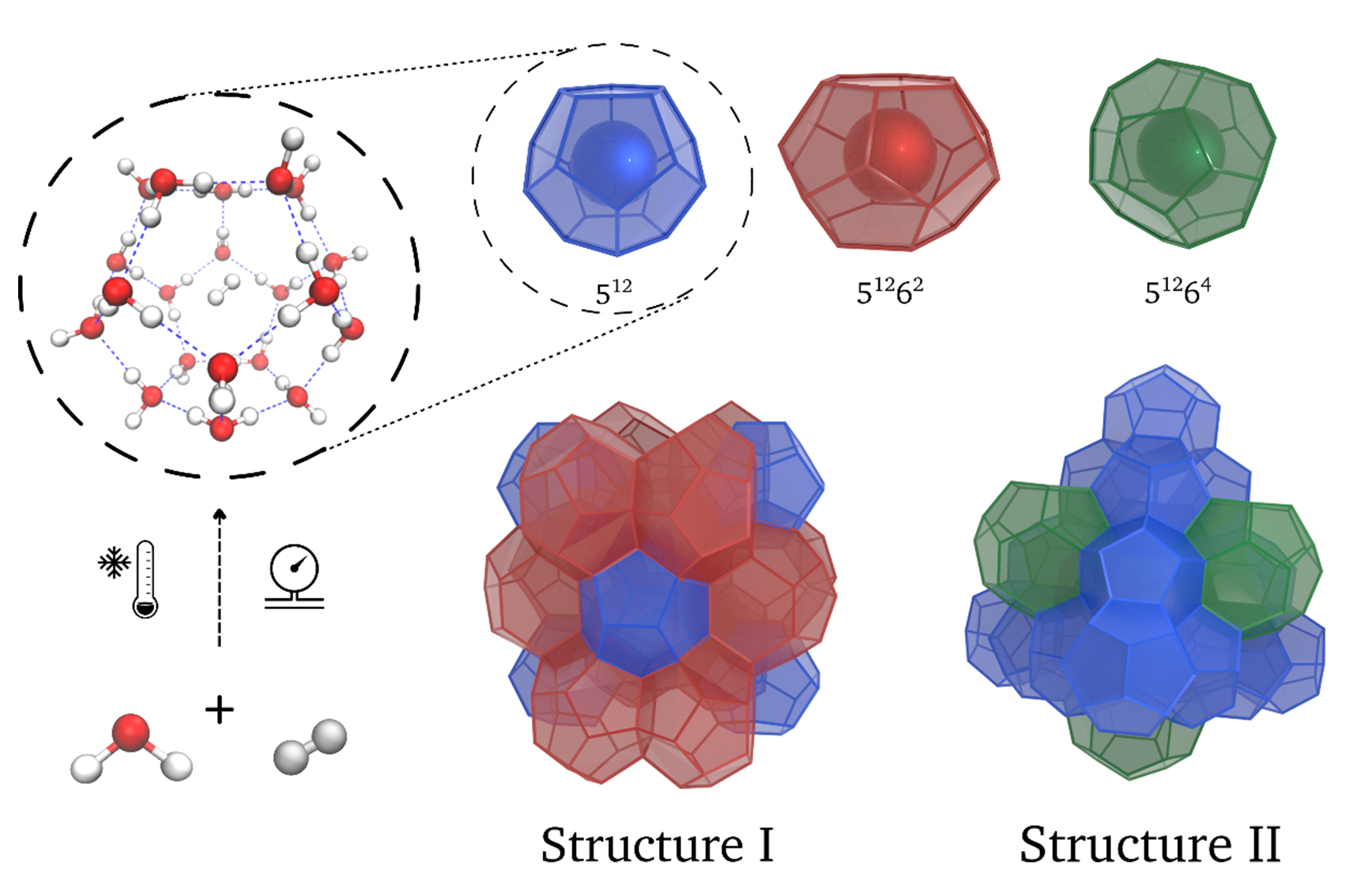
Figure 1: The atomic structure of two types of clathrates: structure I (or methane) clathrates, and structure II (or hydrogen) clathrates.
Goal
In this thesis, we aim to shed light on the fundamental interactions determining the flushing process in clathrates in the context of hydrogen clathrates. This molecular diffusion process will be systematically studied in different clathrate structures by contrasting two complementary approaches. In a first approach, the diffusion behavior will be probed by determining the free energy barriers associated with the intercage hopping of hydrogen using enhanced sampling methods. Due to the low temperatures at which clathrates are formed, in combination with the presence of light-weight elements such a hydrogen, the possible influence of a proper description of nuclear quantum effects will also be investigated. In a second approach, the diffusion process will be studied in “real time” via molecular dynamics simulations, in which the atomic nuclei are propagated throughout time by integrating Newton’s equations of motion. The student will of course be actively coached to make him/her acquainted with the advanced simulation techniques and to transfer necessary programming skills needed to perform the research.
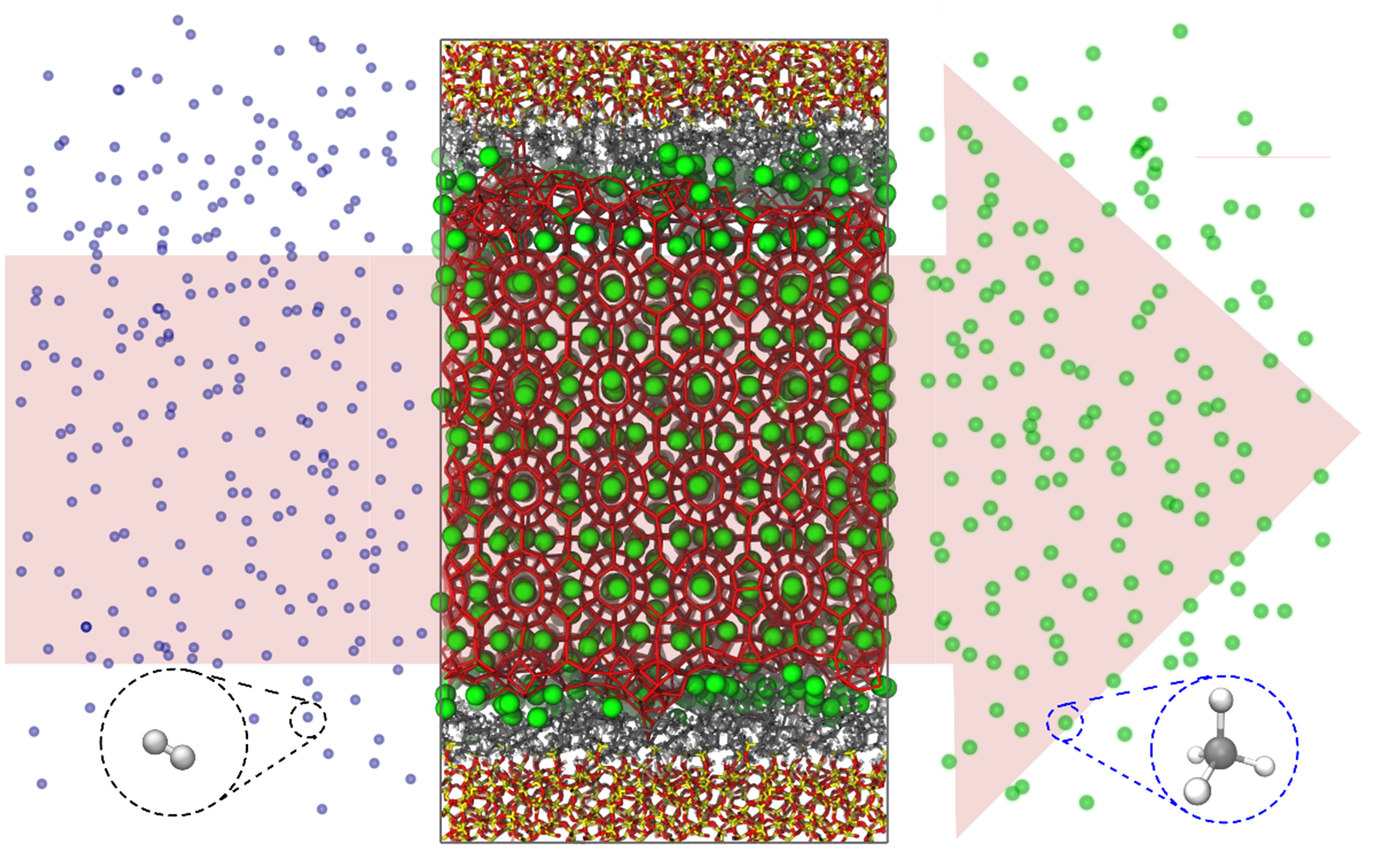
Figure 2: Schematic representation of the flushing process in which methane is flushed out by hydrogen inside a clathrate that is present in a nanoporous material. The impact of the nanoporous material on this process is clear from the preferential organization of methane near the clathrate / nanoporous material interface.
The two approaches outlined above will first be adopted to examine the bulk clathrates of Figure 1. Subsequently, the clathrate structures will be incorporated in nanoporous materials to determine the influence of the presence of an external surface. Special care will be taken to select a diverse set of nanoporous materials by varying the structure and hydrophobicity of the internal pore surfaces, as these parameters were found to critically affect the organization of clathrates and methane in the material.3 Given that these complex hybrid systems contain many thousands of atoms, the use of GPU-accelerated software will be indispensable to push the computational resources required to simulate these systems; these simulations will therefore be performed on our high-performance GPU infrastructure. In this simulation setup, the flushing process of replacing methane by hydrogen can be emulated by applying a concentration gradient of these molecules, as shown in Figure 2, allowing us to quantify the impact of confinement on the flushing process. This, in turn, will help us to understand how the flushing process takes place and how it can be sped up whilst retaining the clathrate structure, which is vital information to bring hydrogen clathrates closer to economic reality.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]Keywordshydrogen storage, clathrates, hydrogen bonding, Diffusion, Molecular simulation, Nuclear quantum effectsReferences
1A. Gupta, G.V. Baron, P. Perreault, S. Lenaerts, R.-G. Ciocarlan, P. Cool, P.G.M. Mileo, S.M.J. Rogge, V. Van Speybroeck, G. Watson, P. Van Der Voort, M. Houlleberghs, E. Breynaert, J.A. Martens, J.F.M. Denayer, Energy Storage Materials 41: 69, 2021.
2J.R. Cendagorta, H. Shen, Z. Bačić, M.E. Tuckerman, Adv. Theory Simul. 4: 2000258, 2021.
3P.G.M. Mileo, S.M.J. Rogge, M. Houlleberghs, E. Breynaert, J.A. Martens, V. Van Speybroeck, J. Mater. Chem. A 9: 21835, 2021.
