High-throughput screening of 2D perovskites for game-changing photovoltaics
High-throughput screening of 2D perovskites for game-changing photovoltaics
Promotor(en): V. Van Speybroeck /20SPEC05 / SpectroscopyProbleemstelling:
Perovskites are a broad class of materials which have the same type of crystal structure as CaTiO3, which was discovered in the early 20th century. They were named in honour of the Russian Count, Lev A. Perovski. Perovskites have ABX3 as chemical formula, with A and B cations and X an anion. There is a large and chemically diverse set of constructable perovskites, which can therefore be designed to exhibit a variety of exciting properties such as ferro-electricity (spontaneous polarization), magnetism and superconductivity.
One particular class of perovskites, metal halide perovskites (MHPs), are now under world-wide investigation for an entirely different reason: they can be applied for photovoltaic (PV) purposes [1]. The most prominent MHP currently used is methylammonium (MA) lead iodide (MAPbI3), where the organic MA cation is encaged in a negatively charged lead iodide framework. In the last decade, the efficiency of perovskite-based solar cells has risen extraordinarily, from 3.8 % to 25.2 %, reaching an efficiency comparable to commercially produced silicon solar cells. Given their easier synthesis, direct band gap, lower cost, and tunable properties, MHPs have the potential to revolutionize the PV industry.
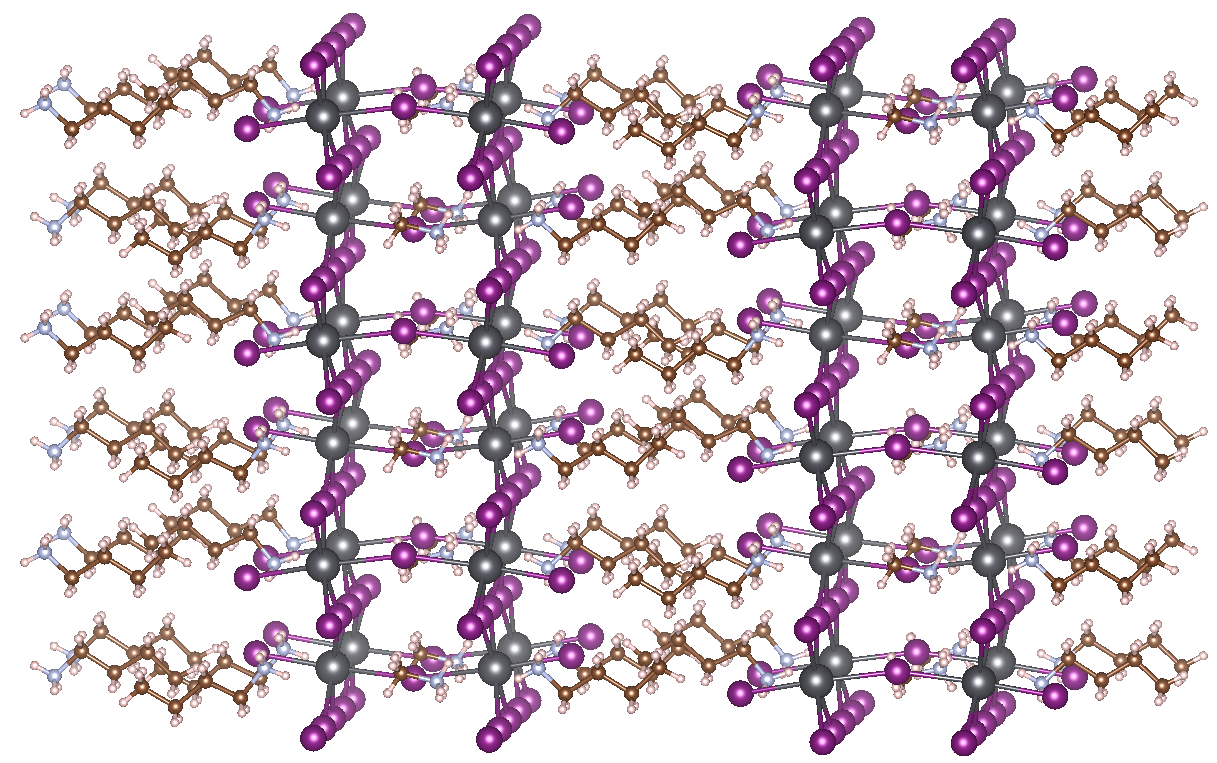
While companies like Oxford PV are already investing to produce perovskite-based solar cells, MHPs still have stability issues. The most important concern is the low resistance of MHPs to moisture. Water easily penetrates the lead iodide cages and degradates the perovskite crystal structure. A possible solution is to use inorganic MHPs (e.g., replacing MA with Cs), although this destabilizes the wanted perovskite phase at room temperature to a metastable phase that will eventually transform to the stable non-perovskite phase with less interesting opto-electronic properties. At the center for molecular modeling (CMM) we recently collaborated with the Hofkens group in Leuven, this synergetic study lead to a novel method to stabilize the black phase [2]. However, long term stability for CsPbI3 is still not achievable. Another solution is to use so-called Ruddlesden-Popper MHPs. These special forms of MHPs consist of 2D layers of traditional perovskite material, interleaved with cations [3]. The most prominent example is MAPbI3 interleaved with n-butylammonium (BA) (see figure). The organic tails extending from the perovskite layers effectively keep out water, explaining the increased resistance to moisture. Thus far, the best available 2D MHP produced has an efficiency of 18%, but tuning the used organic and inorganic components of the structure should make it possible to increase this efficiency further.
Doelstelling:
The solar cell efficiency of a material is related to its electronic structure, as the band gap forms an upper limit for the wavelengths that can be absorbed and the effective electron masses determine the mobility of the charge carriers. Computationally, the electronic structure of 2D MHPs can be calculated using density-functional theory (DFT). DFT is a quantum physical method that allows describing the electron behavior in materials at an acceptable cost. You will be able to employ the VASP package for which there is extensive expertise at the CMM.
When considering the current state of the art in 2D MHPs, the best way to improve the efficiency of these PV materials even further is to modify the spacer cations that separate the perovskite layers. To this end, the band gap of the spacer cation layer must be tuned to the energy of visible light and the energy levels of both layers must be aligned in such a way that they allow efficient charge transfer. However, there are two challenges related with the search for this ideal 2D MHP. (1) It has been shown that the description of the band structure of lead iodide perovskites requires the inclusion of self-energy corrections and spin-orbit coupling, which result in very heavy calculations for large structures. (2) The set of possible 2D MHPs is huge due to the large freedom of choice for the spacer cations but also for the A, B, and X components of the perovskite layer.
Exploratory work showed that there is little to no coupling between the quantum mechanical properties of the perovskite and spacer cation layer. Therefore, the goal of this master thesis is to construct an alignment scheme in order to predict the electronic structure of the 2D MHP from the electronic structures of both layers, a procedure that we coined orthogonal electronic structure engineering (OESE). Using the OESE procedure, not only the number of necessary calculations but also the computational cost is reduced substantially, i.e., a high-troughput screening becomes possible. For the construction of an alignment scheme you can get inspiration from recent work done in our group on metal-organic and covalent organic frameworks [4], although 2D MHPs will pose additional challenges due to their charged components and 2D nature. Furthermore, the accuracy of the alignment method and how it gets effected by incorporation of self-energy corrections and spin-orbit coupling should be assessed via comparison with the electronic structure of the complete 2D MHP.
For our experimental partners it is very interesting to know if the most promising 2D MHPs can be synthesized. Therefore, if time allows it, we need to construct the 2D MHPs and asses its stability by calculating formation energies and deformation modes. Analyzing these results could lead to extra selection criteria for 2D MHPs.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]KeywordsPerovskites, Density functional theory, photovoltaics, electronic structure, nanoscale modelingReferences
[1] W.-J. Yin et al., J. Mater. Chem. A 3, 8926-8942 (2015)
[2] Steele et al., Science 365, 679-684 (2019)
[3] Lai et al., J. Am. Chem. Soc. 140, 11639−11646 (2018)
[4] De Vos et al., J. Mater. Chem. A 7, 8433-8442 (2019)
