Forming porous ice in nanoporous materials: Nucleation of clathrates to store and transport natural gas
Forming porous ice in nanoporous materials: Nucleation of clathrates to store and transport natural gas
Promotor(en): V. Van Speybroeck /25627 / Nanoporous materialsBackground and problem
Natural gas (NG), which mainly consists of methane, is known to be one of the cleanest alternatives for energy harvesting. However, its extensive use is severely hampered by its high transportation costs, mainly due to its low density when stored in its pure form. NG storage in the form of methane clathrates has been suggested as one of the most promising alternatives for NG transportation, mainly due to its environmentally friendly constituents and low safety risk. [1] Clathrates are ice-like structures formed by the envelopment of small apolar molecules by hydrogen bonded water molecules, forming highly ordered networks with specific topologies (Figure 1).
Methane clathrates can be formed naturally, for instance under high pressures and low temperatures on the ocean floor. However, these difficult formation conditions and the sluggish kinetics severely limit the synthesis of methane clathrates from water and methane on an industrial scale (Figure 1, left). Forming clathrates inside hydrophobic nanoporous materials is one of the most promising alternatives to improve clathrate formation. By repelling water from its hydrophobic internal surface, these materials allow for a higher methane-water contact area and gas uptake, and considerably favor clathrate nucleation and growth (Figure 1, right).
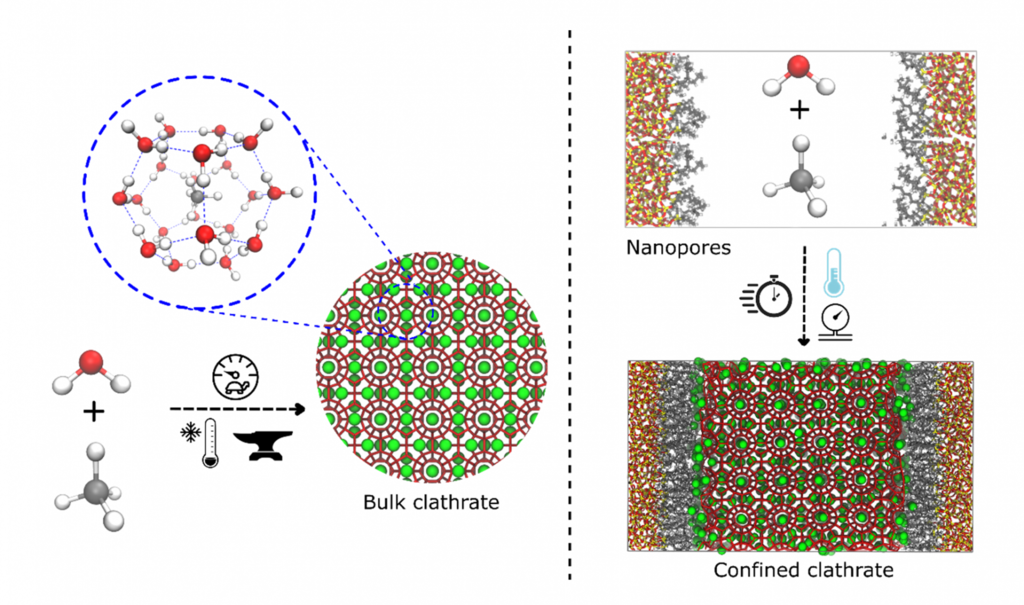
Figure 1. Towards a more efficient clathrate generation: use of nanoporous materials (right) improve the synthesis conditions of clathrates compared to the conventional sluggish and inconvenient process (left).
Nevertheless, the enormous diversity of available nanoporous materials highlights the need to rationally select optimal materials for clathrate storage. This rational selection depends on the development of theoretical models describing the nucleation of clathrates in confinement, starting from a quantum mechanical description or classical description of the potential energy surface. Recently, molecular dynamics simulations have had considerable success in detailing the clathrate nucleation in the bulk state. [2,3] Roughly speaking, such a homogeneous nucleation mechanism is dictated by the concerted water arrangement around a guest molecule leading to the formation of isolated nuclei (“blobs”) which further grow into a full clathrate structure (Figure 2a).
However, the clathrate nucleation in solid surfaces is a more complex problem. Early efforts involving hydrophilic substrates (e.g. silica) have shown that the clathrate nucleation occurs in an intermediate zone between the center of the pore and its surface, with buffering water layers (Figure 2b). [4] Yet, since recent experimental and theoretical accounts revealed the unintuitive hydrophobicity of clathrate surfaces, [5] the nucleation of clathrates in hydrophobic surfaces is assumed to occur differently.
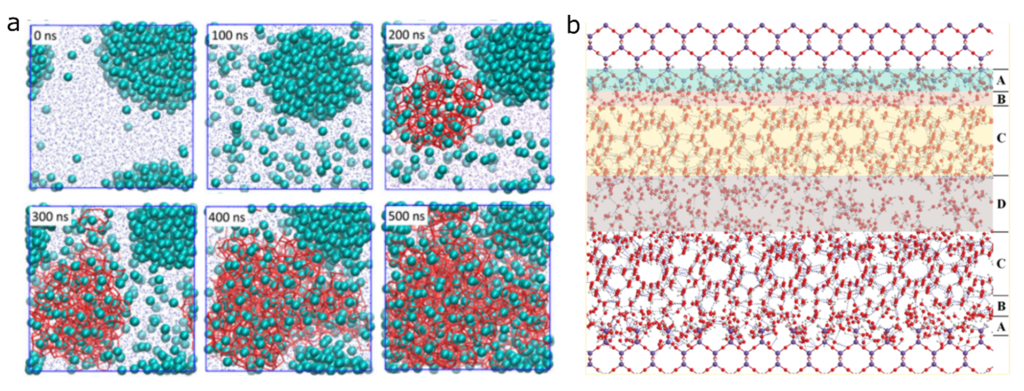
Figure 2. (a) Blob mechanism of clathrate growth. (b) Clathrate formation next to a hydrophilic surface (adapted from Refs. 2 and 4).
Goal
The aim of this proposal is (i) to extend the knowledge on the nucleation and growth mechanism of clathrates within pores containing hydrophilic surfaces and (ii) to explore the nucleation pathway in hydrophobic porous materials that are currently being synthesized within the context of the ARCLATH moonshot project. This national network aims at developing new clathrate-promoting materials in a concerted effort involving academic and industrial partners.
This will be achieved with the use of GPU-accelerated massive classical molecular dynamics (MD) simulations applied to realistic molecular models of confined water/methane mixtures previously constructed in our group. Such an in-silico clathrate formation will also be accelerated by applying the Transition Path Sampling (TPS) methodology. [6,7] TPS yields an ensemble of unbiased molecular dynamics trajectories connecting initial and final configurations by performing a random Monte Carlo walk in the trajectory space. This approach focusses on the rare events associated with the clathrate formation and avoids the unnecessary induction times typical of classical MD calculations. With such characteristics, TPS has recently proved to be particularly suitable for in-depth descriptions of clathrate formation. [3,8] This computational protocol will be carried out using the OpenMM code together with the OpenPathSampling library. These computational platforms were specifically designed for high-demanding computations and will allow to efficiently compute and describe the large systems involved in this proposal.
With the results developed in this project we expect not only to contribute to a further understanding of the underlying hydrate formation, but we will also try to pave the way for the development of new materials capable of efficiently promoting and storing clathrates.
Within the scope of this work, the student will get acquainted with current molecular dynamics methods and advanced sampling techniques, and learn to choose the appropriate level of theory to build models and to investigate these challenging nano-systems.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]Keywordsclathrate nucleation, materials development, gas storage, energy storageReferences
1. Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285, doi:10.1016/j.apenergy.2018.02.059.
2. Li, L.; Zhong, J.; Yan, Y.; Zhang, J.; Xu, J.; Francisco, J.S.; Zeng, X.C. Unraveling nucleation pathway in methane clathrate formation. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 24701–24708, doi:10.1073/pnas.2011755117.
3. Arjun; Berendsen, T.A.; Bolhuis, P.G. Unbiased atomistic insight in the competing nucleation mechanisms of methane hydrates. Proc. Natl. Acad. Sci. 2019, 116, 19305–19310, doi:10.1073/pnas.1906502116.
4. Bai, D.; Chen, G.; Zhang, X.; Wang, W. Microsecond Molecular Dynamics Simulations of the Kinetic Pathways of Gas Hydrate Formation from Solid Surfaces. Langmuir 2011, 27, 5961–5967, doi:10.1021/la105088b.
5. Bertolazzo, A.A.; Naullage, P.M.; Peters, B.; Molinero, V. The Clathrate–Water Interface Is Oleophilic. J. Phys. Chem. Lett. 2018, 9, 3224–3231, doi:10.1021/acs.jpclett.8b01210.
6. Bolhuis, P.G.; Chandler, D.; Dellago, C.; Geissler, P.L. TRANSITION PATH SAMPLING: Throwing Ropes Over Rough Mountain Passes, in the Dark. Annu. Rev. Phys. Chem. 2002, 53, 291–318, doi:10.1146/annurev.physchem.53.082301.113146.
7. Swenson, D.W.H.; Prinz, J.-H.; Noe, F.; Chodera, J.D.; Bolhuis, P.G. OpenPathSampling: A Python Framework for Path Sampling Simulations. 1. Basics. J. Chem. Theory Comput. 2019, 15, 813–836, doi:10.1021/acs.jctc.8b00626.
8. Arjun, A.; Bolhuis, P.G. Rate Prediction for Homogeneous Nucleation of Methane Hydrate at Moderate Supersaturation Using Transition Interface Sampling. J. Phys. Chem. B 2020, 124, 8099–8109, doi:10.1021/acs.jpcb.0c04582.
