First-principle study of olefin formation pathways in a complex molecular environment for the direct conversion of CO2 on zeolite catalysts
First-principle study of olefin formation pathways in a complex molecular environment for the direct conversion of CO2 on zeolite catalysts
Promotor(en): V. Van Speybroeck /25473 / Nanoporous materialsBackground and problem
Reducing atmospheric CO2 emissions to mitigate the impact of global warming is one of the major challenges for the chemical industry. Currently, the development of carbon capture and usage (CCU) technologies to directly transform the captured CO2 into valuable chemicals or fuels receives a lot of interest. Promising results in the CO2 reductive valorization are obtained with a combination of conventional metallic catalysts and acid zeolites. [1-3] The metallic catalyst is used to convert CO2 into CO, which is in turn converted into hydrocarbons through classical Fischer-Tropsch synthesis. The acid zeolite increases the light olefin and aromatics yield by transformation of these hydrocarbons through cracking, aromatization, isomerization,… reactions. Alternatively, the metallic catalyst can convert CO2 into methanol, which is further processed in the zeolite framework through the methanol-to-hydrocarbons (MTH) chemistry. In order to optimize these bifunctional catalysts, thorough fundamental insight into the governing reaction mechanisms is essential.
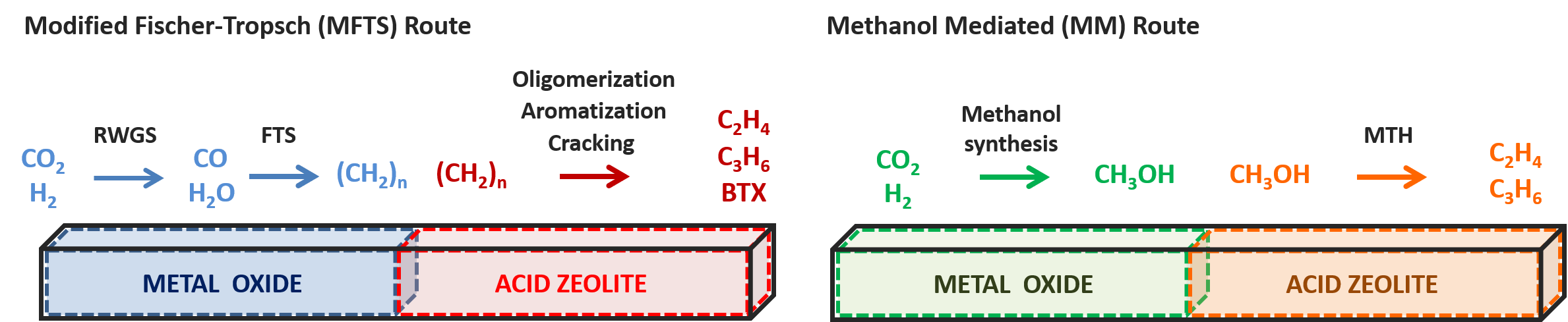
Figure 1. Illustration of two pathways for conversion of CO2 into light olefins on a metal oxide-zeolite bifunctional catalyst.
One of the main challenges is to tune the product distribution towards the desired products such as light olefins or aromatics. This is complicated by the many competitive reactions (cracking, hydrogenation, alkylation …) taking place simultaneously and by the presence of CO, CO2, H2O and H2 in the zeolite pores which can affect the stabilization of crucial reaction intermediates and the governing reaction mechanisms. Recent experimental studies pointed to the co-existence of multiple unexplored olefin producing pathways. Next to the conventional MTH reaction cycles also C-C coupling reactions involving important intermediates such as ketenes or surface acetates would be operational. [4-5] However, the precise role of these reactive, short-lived species on the selectivity of the formed olefinic and aromatic hydrocarbons is not fully understood.
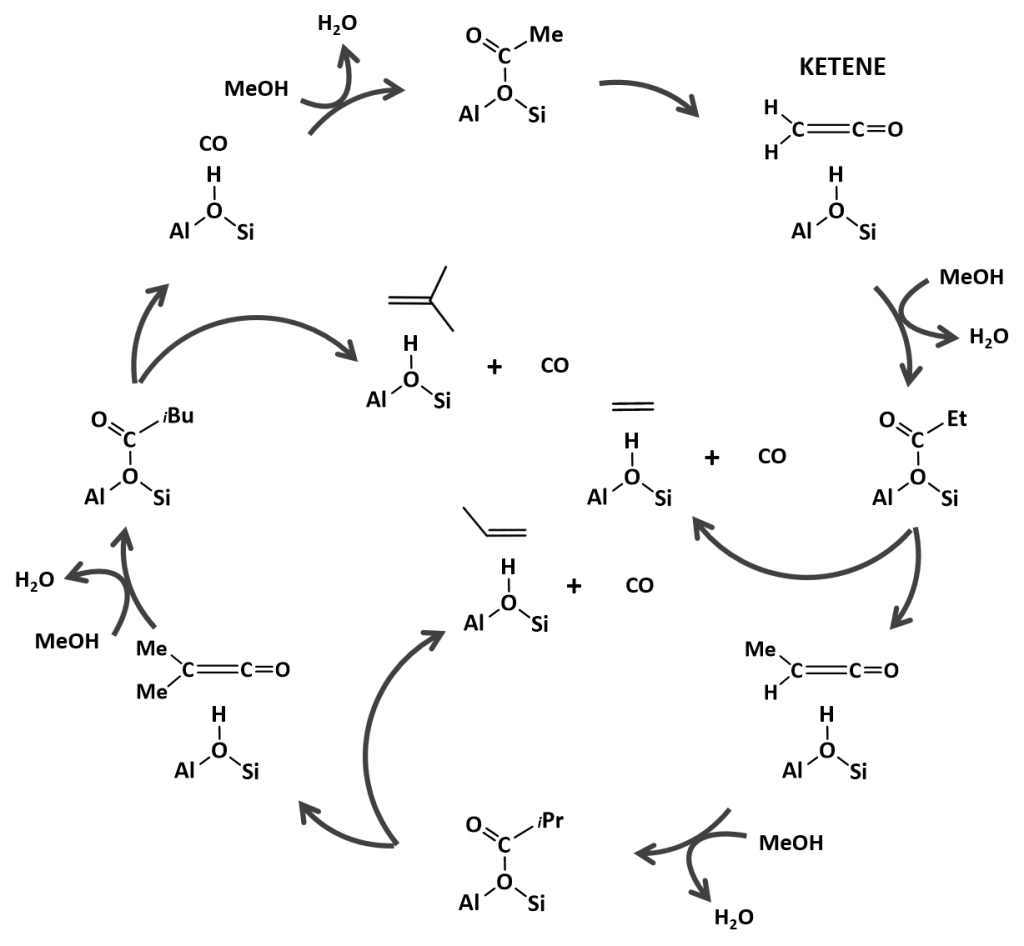
Figure 2. Proposed ketene-based mechanism for the formation of light olefins during the zeolite catalyzed MTH process. [4]
Goal
The goal of this master thesis is to investigate how elementary C-C bond formation reactions are affected by the complex molecular environment and the specific zeolite environment. First, you will build zeolite models which can realistically represent the actual loading of CO, CO2, H2, H2O,… in the confined catalyst pores. To this end, thermodynamic techniques will be applied to estimate the occupation of these guest molecules in the zeolite at operating conditions. Afterwards, the stability of various intermediates (ketene, surface acetates,…) will be evaluated in these models. You will simulate key reaction pathways involving these intermediates to assess the critical influence of the presence of CO, H2O,... in the reaction mixture on the reaction mechanism. To discriminate between competitive pathways and to take into account the complex molecular environment, first-principle molecular dynamics simulations at operating conditions will be performed. As the role of the zeolite is crucial for finetuning the product distribution towards either a high light olefin or aromatics yield, you will consider zeolites with different pore topologies in this study. Ultimately, this will yield insight in the role of the zeolite topology and molecular environment on the light olefin formation mechanisms and will assist in improving the selectivity of CO2 conversion processes.
The Center for Molecular Modeling has ample experience in modeling of zeolite catalysis with advanced simulation techniques. The proposed topic is situated in a very active research field. The student will be actively coached to get acquainted with the multitude of available techniques to tackle the proposed problem. The CMM has access to large scale computational infrastructure for studying this complex system. The student will be actively involved in the running collaboration with prof. Jorge Gascon (KAUST) on this topic.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, CO2-to-hydrocarbonsReferences
[1] A. Dokania et al., ACS Energy Lett. 4 (2019) 167-176.
[2] J. Wei et al., Nat. Comm. 8 (2017) 15174.
[3] Q. Zhang et al., Adv. Mater. 32 (2020) 2002927.
[4] A.D. Chowdhury and J. Gascon, Angew. Chem. Int. Ed. 57 (2018) 14982-14985.
[5] A. Ramirez et al., ACS Catal. 9 (2019) 6320-6334.
