First-principle kinetic study on active olefin formation cycles during methylation conversion in industrially important catalysts
First-principle kinetic study on active olefin formation cycles during methylation conversion in industrially important catalysts
Promotor(en): V. Van Speybroeck /15_NANO07 / Nanoporous materialsThe conversion of methanol into hydrocarbons (MTH) or olefins (MTO) received a lot of attention during the last decades as methanol can be produced from natural gas, biomass or coal. The conversion is catalyzed by zeolite or zeotype materials. It has been found that the inorganic catalyst framework and occluded hydrocarbons catalyzed the MTO reactions. Moreover, a couple of catalytic cycles operate simultaneously during olefin formation, resulting in a very complex reaction mechanism which is currently still not fully understood. Many factors as framework topology, acid strength and process conditions determine which cycle dominates the product formation. Detailed molecular-level insights into the complex reaction network can be obtained by molecular simulations.
Both aromatics and aliphatics – the so-called hydrocarbon pool compounds – were found to play a crucial role in the formation of olefins from methanol as indicated in Figure 1. However, many theoretical studies point out that some of these cycles still exhibit very high reaction barriers, indicating that the real operating mechanism isn’t discovered yet. Even for the two industrially used catalysts, H-ZSM-5 and H-SAPO-34, the active routes are not yet unraveled to date. There is an urgent quest to reveal the nature of the active routes to assist the experimentalists in tuning the catalyst towards optimal performance.
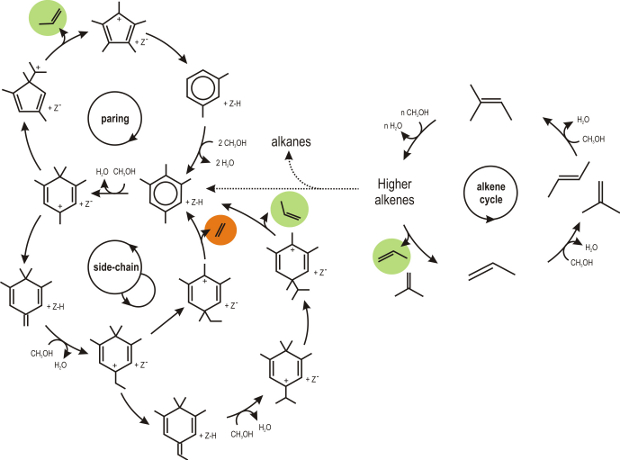
Figure 1. Overview of the proposed mechanisms for olefin formation during methanol conversion.Goal In this thesis project the goal is to discover new hydrocarbon pool compounds and find new low-barrier reaction paths that lead to olefin formation. The research will focus on active routes within two industrially used catalysts, i.e. H-ZSM-5 and H-SAPO-34. For this purpose, first principle calculations will be applied. First, advanced molecular dynamics techniques will be applied that allow the discovery of new reaction intermediates and transition paths. Next, techniques will be applied that are able to determine accurate kinetics of these newly constructed reaction cycles. The main focus will be on reactions involving intramolecular rearrangements of cyclic carbenium ions, in which methanol is not playing a direct role. The Center for Molecular Modeling has ample experience with these recently developed techniques and can provide sufficient computational resources to execute this research project. Furthermore the research is performed in close collaboration with prominent experimental partners with whom the CMM has built solid research collaboration over the last years. This ensured valuable input on some intermediates discovered theoretically and vice versa. The student will be involved in work discussions with our experimental partners.
The proposed topic is challenging and requires technical skills, creativity and chemical insight. Furthermore there will be a close interaction with the researchers working on spectroscopy in nanoporous materials (headed by Prof. K. Hemelsoet).
- Study programmeMaster of Science in Chemical Engineering [EMCHEM], Master of Science in Chemistry [CMCHEM]KeywordsHeterogeneous Catalysis, Chemical kinetics, Computational applications

