Effect of defect coordinating species on the reactivity of Metal-Organic Frameworks (MOFs)
Effect of defect coordinating species on the reactivity of Metal-Organic Frameworks (MOFs)
Promotor(en): V. Van Speybroeck /15_NANO04 / Nanoporous materialsMetal-Organic Frameworks (MOFs) are - just as zeolites - ordered nanoporous materials with pore sizes between 0.5 and 2 nm. In contrast to zeolites, which have a purely inorganic character, MOFs constitute a class of porous materials that exhibit a truly hybrid character, since both inorganic and organic moieties are needed to build up their framework. While catalytic conversions and separations are the most frequently used industrial applications of zeolites, future MOF applications lie more in adsorption, storage and separation of gas mixtures (H2, CO2 or CH4), and nowadays their importance in catalysis is steadily increasing. Although catalysis is potentially one of the most important applications of these exciting class of materials, design of suitable MOFs for catalytic applications remains a challenge to be explored.
With the aid of molecular modeling and experimental work, we unraveled that the active metal sites in Zr-terephthalate (UiO-66 type materials [1], Figure 1(a)) need to be partially decoordinated to be active [2]. We have previously studied how linker defect sites can be coordinated in the UiO-66 framework, for example with loose hydroxyl groups, chlorine groups [3]. As the effect of such linker defect terminations on catalysis has not been thoroughly investigated from both experimental and computational point of view, an investigation is warranted.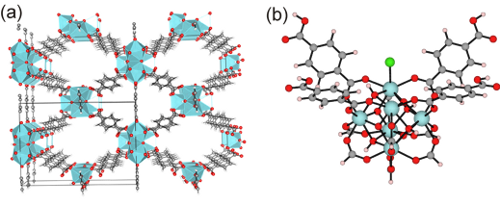
Figure 1: (a) periodic model of the UiO-66 with one defect present, (b) cluster model of chlorine coordination to a defect site.Coordinating groups are typically removed during a post synthesis heat treatment, however, can be recreated by exposing the framework to certain solvents. There are many examples of interesting Zr-coordinating species, such as HO- (H2O), HS- (H2S), F- (HF), Cl- (HCl), etc. The molecules from which they originate are dissociated on the Lewis acid defect site, forming on the one hand a Brønsted proton and on the other hand a Brønsted base that coordinates to the Zr-site. Figure 1(b) illustrates nicely the formation of a Zr-Cl species, which forms spontaneously when HCl is put on the cluster model.
Goal Within this thesis proposal it is the intention to study the Oppenauer oxidation reaction on the genuine UiO-66 material and various related materials in which defects have been created. To that end a thorough investigation needs to be performed on the nature of various possible active sites with and without defect coordinating species. The CMM has ample expertise with various modeling tools to perform the envisaged work program. A variety of cluster and periodic models will be used to model the catalyst and static and advanced molecular dynamics tools will be used to study the reactive pathways. The chemical properties of the defect sites will be investigated such as the the proton affinity and the charge distribution on the reactive atoms (different partitioning schemes are already implemented and available as CMM-code). One parameter to compare the activity of the various models, can be the protonation free energy barriers of geraniol.
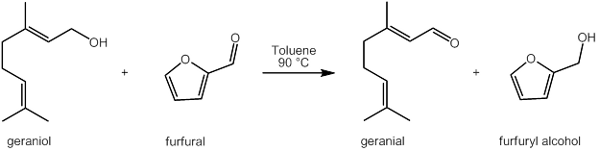
Figure 2: Oppenauer oxidation reactionExisting quantum chemical methods and software packages will be applied to search for reaction paths on these clusters that can explain the product distribution (or selectivity). The end goal is to get insight in the activity modulating behavior of defect coordinating species within UiO-66 type materials and predict activity of these tailor made (defect engineered) Zr-sites for a variety of other reactions.
The research will be performed in the framework of a larger concerted action to simulate chemical transformations at operating conditions. The Center for Molecular Modeling has built up vast expertise in these advanced simulation techniques and collaborates on the subject with leading experimental and theoretical partners. It is the intention to involve the student actively in the work discussions with our collaborators. The CMM has access to sufficient computational resources to execute this research project. The proposed topic is challenging and requires technical skills, creativity and chemical insight.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]References
[1] J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability,Journal of the American Chemical Society, 2008, 130, 13850.
[2] F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck, D.E. De Vos, Electronic Effects of Linker Substitution on Lewis Acid Catalysis with Metal-Organic Frameworks, Angewandte Chemie-International Edition, 2012, 51, 4887.
[3] M. Vandichel, J. Hajek, F. Vermoortele, D. De Vos, M. Waroquier, V. Van Speybroeck, Active site engineering in UiO-66 type metal-organic frameworks by intentional creation of defects: a theoretical rationalization, CrystEngComm, 2015, 17 (2), 395-406.


