Combining experimental and computational research to investigate the properties of Metal-Organic Frameworks
Combining experimental and computational research to investigate the properties of Metal-Organic Frameworks
Promotor(en): V. Van Speybroeck, H. Vrielinck /16SPEC02 / SpectroscopyMetal-organic frameworks (MOFs) recently attracted much attention in the scientific community due to their various applications and astonishing chemical and physical properties. These materials consist of metal oxides connected to each other through organic ligands, resulting in crystalline and nanoporous structures with a large surface to volume ratio. The size of the nanopores is mainly determined by the choice of the organic ligand. Depending on the specific choice of metal oxide and organic ligand, these MOFs can have applications in chemical catalysis, storage, detection and separation of gases, optical and semi-conducting materials. Furthermore, some MOFs exhibit an extraordinary transformation between distinct crystal structures. Such transformation can be induced through external stimuli such as temperature, pressure, interactions with guest molecules, light, … This phenomenon is usually denoted as breathing.
Due to the chemical versatility with which these materials can be synthesized, the number of possible MOFs is tremendously high. As a result, it is impossible to scan all possible candidates experimentally for a certain application. Therefore, to fully expose the full potential of these extraordinary materials, scientist typically apply both experimental and computational techniques, combining the strengths of both techniques. For example, computed equilibrium geometries can be compared with the results of diffraction experiments, while lattice vibrations and the electronic band structure are directly related to various spectroscopic techniques.
Goals
In this thesis subject, we focus on a special class of MOFs, containing aluminum or vanadium oxides and pores with the shape of long rectangular channels. The pristine materials are the so-called Mil-53(Al) (metal-oxide = [Al-OH]; organic ligand = [benzene dicarboxylate, BDC]) and Mil-47(V) (metal-oxide = [V=O]; organic linker = [BDC]). Depending on the mechanical pressure, temperature and possibly the interactions with guest molecules, Mil-53(Al) can be either in a state with large open pores or a state with narrow closed pores. Although Mil-47(V) does not exhibit transformations between such lp and np states under influence of the interaction with guest molecules, it does show such transformations when mechanical pressure is applied. By means of an investigation using mixed metal frameworks (eg. Mil-53(Al) doped with V), we wish to obtain a deeper understanding of the mechanism behind these breathing transitions. Structures with different linkers (naphthalene dicarboxylate and biphenyl dicarboxylate) will also be investigated.
X-ray diffraction is the standard technique to determine the crystal structure, hence this method can be used to detect breathing transitions. However, such transitions also result in small changes in the lattice vibrations and electronic structure of the paramagnetic ions (eg. V4+). These changes can be traced in situ with far-infrared (Far-IR) spectroscopy and electron paramagnetic resonance spectroscopy (EPR). Recently, the application of EPR gave rise to unexpected results that are not yet fully understood, while the application of Far-IR on MOF structures is unique thus far.
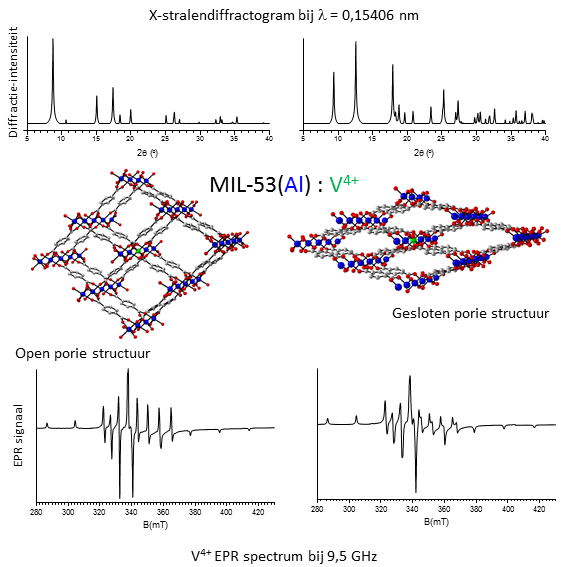
By means of molecular modeling, one can investigate the breathing behavior on a molecular level. Using quantum mechanical calculations based on Density Functional Theory (DFT), one can compute the geometric and electronic structure of these materials. As a result, we can compute several observables such as the XRD pattern as well as the IR spectrum and compare it with the experimental results. The breathing transitions between the large pore and the narrow pore can be simulated as well, however, quantum mechanical calculations are usually not feasible for such large length/time scale. As a result, one usually applies so-called force fields in the context of breathing MOFs. A force field is a mathematical expression for the interaction between the atoms in the molecule (the electrons are not described explicitly) based on simple spring models. The unknown parameters occurring in the energy expression, such as the force constant and rest length of the springs, can be estimated either from experimental or quantum mechanical input. Using these force fields, one can perform molecular dynamic simulations that can trace the behavior of metal-organic frameworks through long periods of time, allowing to characterize the breathing transitions.
In this thesis, both experimental work and computational modeling can be applied. Depending on the interest of the student, the focus can be shifted towards one or the other.
The materials will be synthesized at COMOC (Center for Ordered Materials, Organometallics and Catalysis, UGent, prof. P. Van Der Voort), hence this research will be performed in close collaboration with COMOC.
Aspects
Engineering: The student will apply several experimental and computational techniques to characterize new materials and investigate their potential for various applications.
Physics: The student will use several techniques and interpret their results, which requires a fundamental understanding of quantum mechanics, solid state physics and atomic and molecular physics
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) NANO, MODELINGKeywordsMetal-organic frameworks, X-Ray Diffraction, Infrared Absorption Spectroscopy, Electron Paramagnetic Resonance Spectroscopy, Quantum mechanics, Density functional theory

