Chemical kinetics of Lewis-catalyzed reaction within Metal-Organic Frameworks
Chemical kinetics of Lewis-catalyzed reaction within Metal-Organic Frameworks
Promotor(en): V. Van Speybroeck /15_NANO01 / Nanoporous materialsMetal-Organic Frameworks (MOFs) are - just as zeolites - ordered nanoporous materials with pore sizes between 0.5 and 2 nm. In contrast to zeolites, which have a purely inorganic character, MOFs constitute a class of porous materials that exhibit a truly hybrid character, since both inorganic and organic moieties are needed to build up their framework. While catalytic conversions and separations are the most frequently used industrial applications of zeolites, future MOF applications lie more in adsorption, storage and separation of gas mixtures (H2, CO2 or CH4), and nowadays their importance in catalysis is steadily increasing. Although catalysis is potentially one of the most important applications of these exciting class of materials, design of suitable MOFs for catalytic applications remains a challenge to be explored.
With the aid of molecular modeling and experimental work, we unraveled that the active metal sites in Zr-terephthalate (UiO-66 type materials [1]) need to be partially decoordinated to be active [2]. In other words, missing terephthalates create these active sites. New materials were made by inserting electron-withdrawing substituents on the terephthalates and tested for the citronellal cyclization (reaction 1, displayed in Figure 2). Both the catalyst activity and selectivity towards the desired isopulegol product increased significantly. The best result was obtained for nitro-substituents and was further studied by molecular modeling on two types of cluster models (cluster representation in Figure 1).
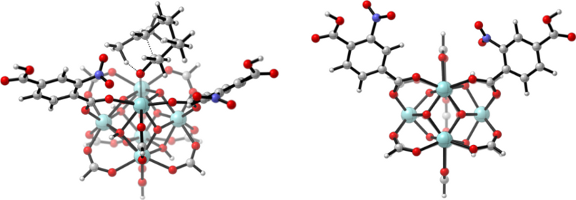
Figure 1: LEFT: side view of UiO-66-NO2 cluster model for the citronellal cyclization; RIGHT: top view on the modeled active site.In this thesis project, we will computationally study other types of reactions such as the Meerwein-Ponndorf-Verley reduction of 4-tert-butylhexanone (reaction 2, Figure 2). For reaction 2 both the carbonyl compound and the alcohol need to be simultaneously activated on the active site. Preliminary experimental work performed at the Centre for Surface Chemistry and Catalysis (COK, KULeuven) shows that Zr-based UiO-66 type of materials may have great activity for such hydrogen transfer reactions. The scientific question to be resolved from molecular modeling point of view consists in unraveling the nature of the active site making these types of reactions possible. Active sites of various substituted UiO-66s will be modeled to provide insight into the detailed reaction mechanism and selectivity of reaction 2 (Figure 2).
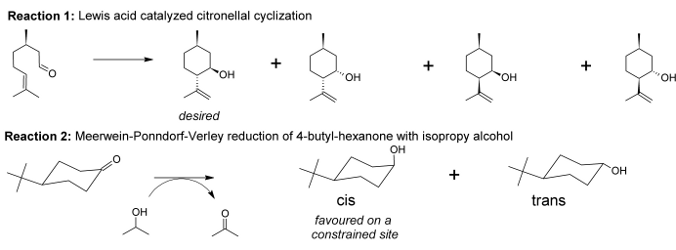
Figure 2: Overview of reactions that can be studied to unravel the active site topology of the UiO-66-X materials.In concert with the thesis student, experimental catalytic results will be interpreted and translated to various cluster models using molecular modeling tools. Then, existing quantum chemical methods and software packages will be applied to search for reaction paths on these clusters that can explain the product distribution (or selectivity). The end goal is to get insight in the selectivity determining factors of the UiO-66-X type materials. If demanded by the student, the topic can be slightly extended by in silico testing of new modifications to alter the activity/selectivity of these new materials.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]References
[1] J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability,Journal of the American Chemical Society, 2008, 130, 13850.
[2] F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck, D.E. De Vos, Electronic Effects of Linker Substitution on Lewis Acid Catalysis with Metal-Organic Frameworks, Angewandte Chemie-International Edition, 2012, 51, 4887.


