Characterizing crucial reaction intermediates of the Selective Catalytic Reduction (SCR) reaction of NOx in CuCHA via Ab Initio Simulations
Characterizing crucial reaction intermediates of the Selective Catalytic Reduction (SCR) reaction of NOx in CuCHA via Ab Initio Simulations
Promotor(en): K. Hemelsoet /15_SPEC08 / Nanoporous materials, SpectroscopyAmmonia-Selective Catalytic Reduction (NH3-SCR) is a well-established strategy for the abatement of NOx gases generated from the combustion of diesel fuel. Metal-exchanged zeolites have been successfully implemented as an effective NH3-SCR catalyst, especially in mobile applications (see Figure 1). The reaction temperatures range from 200 oC to 500 oC, depending on the employed catalyst material. Metal-containing zeolites are considered interesting and effective for this reaction. Moreover, recent studies have demonstrated that Cu is particularly active when exchanged into the SSZ-13 zeolite. This zeolite has the chabazite topology, involving double six-ring building units forming spacious cages which are connected via small 8-ring windows. An additional attractive feature of the Cu-SSZ-13 zeolite is the tendency for Cu ions to reside in either the 6 or 8 ring rendering it an excellent model system for fundamental studies into adsorption and reaction.
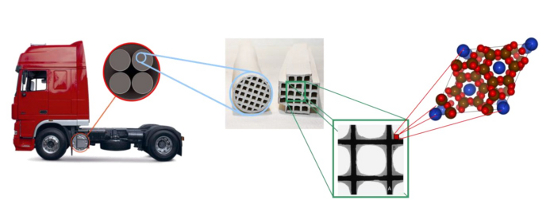
Figure 1. Representation of a zeolite catalyst material used for the catalytic reduction of NOx gases from the combustion of diesel fuel.In spite of the relative simple overall reactions of the SCR-process, the exact details of the underlying reaction mechanism and the corresponding reaction intermediates are still puzzling. In recent studies, we focused on the initial adsorption of the SCR gases, such as NO and ammonia. These adsorptions have been studied using a combination of theory and experiment. In a next step, the influence of water was also assessed, investigating the different locations for the Cu ion. Throughout these studies, vibrational IR spectroscopy is an important characterization technique. In view of this success, IR spectroscopy is also the selected characterization method in this master thesis to obtain vibrational information on a variety of crucial reaction intermediates of the SCR process over Cu-SSZ-13.
The accurate computation of IR spectra of complex systems – such as the Cu-exchanged zeolite materials – requires the use of reliable theoretical methods. A lot of progress was made in this field in recent years. The theoretical IR spectra will be determined based on dynamic simulations using density functional theory. Use of molecular dynamics allows including anharmonic and temperature effects, which are necessary to describe the properties of reaction intermediates of real processes. Figure 2 illustrates the unit cell and computed IR spectrum of a Cu-SSZ-13 containing an ammonia molecule adsorbed at the Cu ion.
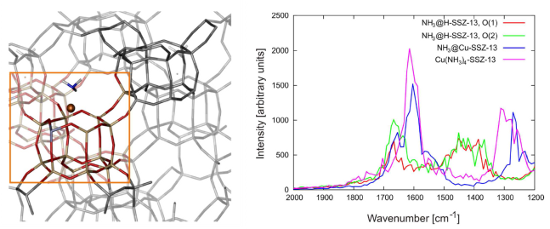
Figure 2. Illustration of a periodic model of Cu-SSZ-13 with ammonia adsorbed at the cation (left). Calculated IR spectra of various adsorption intermediates involving ammonia (right) [from Lezcano-Gonzalez et al., PhysChemChemPhys 16 (2014) 1639.]Aim and objectives Ab initio Molecular Dynamics (AIMD) simulations will be performed on proposed reaction intermediates in the fully periodic Cu-SSZ-13 catalyst material. After getting familiar with the process and the specific molecular modeling techniques, crucial reaction intermediates will be selected and elementary reaction steps involving will be modeled. Suggestions will be taken from the available literature, or will be newly proposed as part of this thesis research. Previous studies report on the important role of temperature for the outcome of the catalytic reaction, and the influence of this process parameter on the geometries, energetics and vibrational properties will be assessed in detail via MD simulations at various temperatures. The MD runs provide direct insight in the dynamic behavior of the reaction intermediates. Importantly, their IR spectra will be computed and analyzed in detail. There will be a close interaction and good coaching with other researchers working on this topic, and depending on the specific interests of the interested student, particular items can be selected for this master thesis research. There will be close interaction with other researchers working on zeolite-catalyzed processes and nanoporous materials (research headed by Prof. V. Van Speybroeck).
The main challenge of this master thesis surely is the complexity of the SCR-process. It is crucial that the interested student applies his/her chemical insight to (1) unravel which reactions and reaction intermediates will dominate in the Cu-SSZ-13 catalyst and (2) unravel the complex IR spectra obtained via the advanced theoretical simulations. This thesis project focusses on an industrially relevant process, for which creativity, chemical insight in the complex process and technical competences need to be combined. It is not necessary to have experience with quantum chemical software codes, this will be learned during the thesis year.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Selective Catalytic Reduction of NOx, Vibrational spectroscopy, Computational applicationsRecommended coursesMolecular Modeling of Industrial Processes


