Chaos vs. Order: The role of thermal contributions to the stability of LaxCe1-xO2-x/2
Chaos vs. Order: The role of thermal contributions to the stability of LaxCe1-xO2-x/2
Promotor(en): S. Cottenier, D.E.P. Vanpoucke /15_MAT11 / Solid-state physicsSince the pioneering work of Zintl and Croatto, La2Ce2O7 has been studied for over half a century. During this time, La2Ce2O7 and more generally the LaxCe1-xO2-x/2 (LCO) compounds have been studied in the context of three-way automotive catalysts, as an ionic conductor in solid oxide fuel cells, and as oxygen sensor. In recent years, LCO has also become of interest as a new material for thermal barrier coatings. Although La2Ce2O7 has been known for a long time, its crystal structure remains a point of discussion.
The two competing models for its crystal structure are the disordered fluorite and the pyrochlore structures. The two structures are closely related and as such difficult to distinguish experimentally. Many ternary oxides with the formula 2B2O7, with +III ions A and +IV ions B, adopt a pyrochlore structure, making the latter a good candidate for the La2Ce2O7 structure. Conversely, in many cases where a pyrochlore crystal structure is observed, the A and B ions are indeed lanthanides and/or transition metals. Starting from the same fluorite lattice the main difference between the two structures boils down to order. Where the pyrochlore structure is highly ordered (with monotype tetragonal cation clusters surrounding the vacancies) the disordered fluorite structure is defined as a fluorite geometry where the cation sites are randomly filled with Ce and La atoms, leading to an average filling of 0.5 Ce and 0.5 La atoms in each cation site. The oxygen vacancies are also distributed randomly leading to an average occupation of 0.875 oxygen atoms per oxygen site.
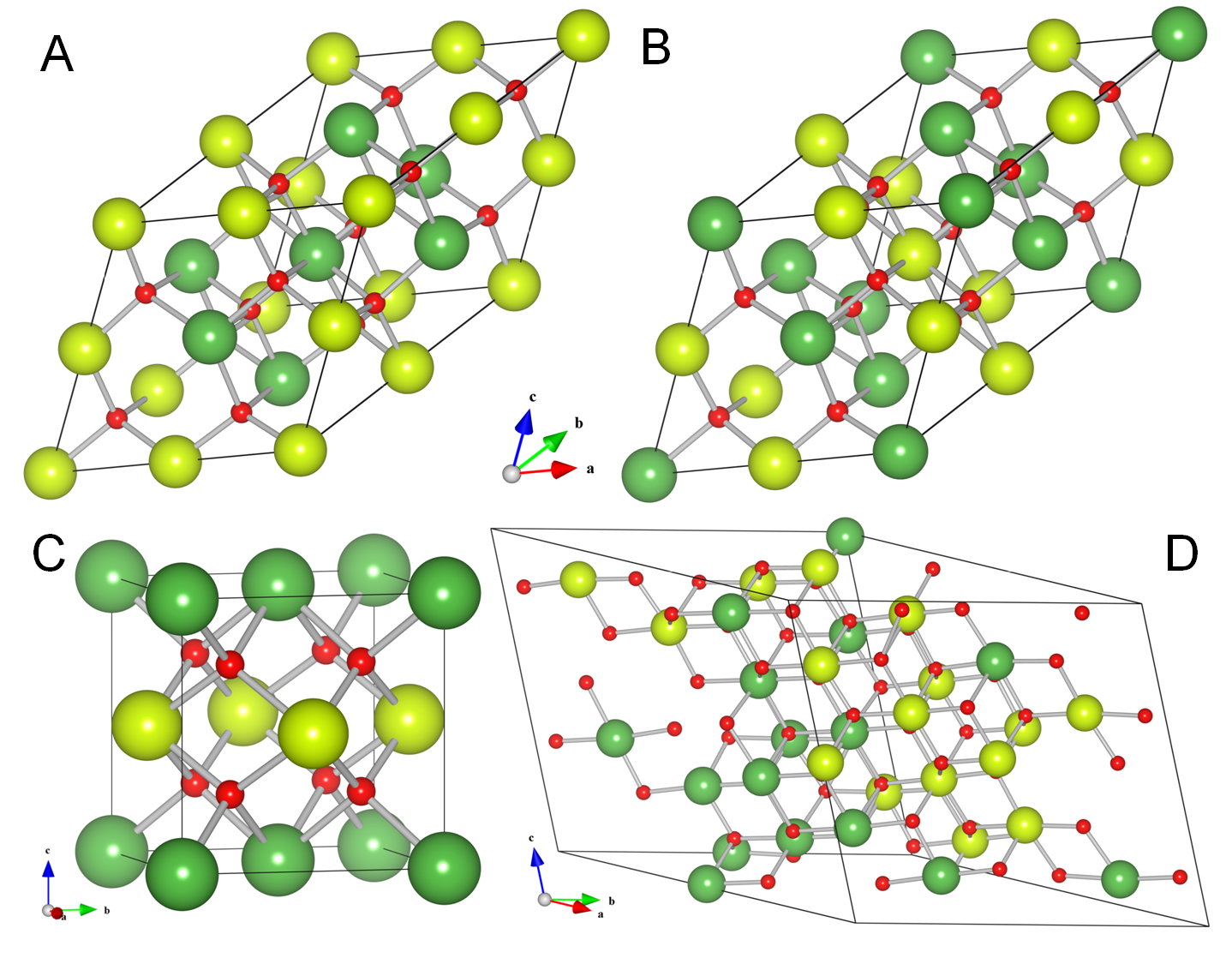
Studies of LCO show an even more interesting picture. Some authors show, using XRD experiments, that the CeO2 fluorite structure is maintained for La concentrations up to x = 0.40 (for La2Ce2O7 x = 0.50), and only at higher La concentrations the pyrochloric ordering of the cations appears. They also observe that the lattice parameter does not expand in a linear fashion as was reported earlier, and assume this reduced expansion is due to the clustering of O-vacancies. Other authors find multiple non-linear regions in the expansion of the lattice parameter and link this behavior to the presence of two phases with different La concentration.
In this thesis topic, the stability of LCO as function of the La concentration will be studied. Comparison of ordered and disordered structures will provide insight into the driving forces for stability. By also including thermal contributions to the energy, comparison with experiment becomes possible and maybe the discussion on the ground state crystal structure can finally be settled.
Interested students will be trained in the use of the required quantum mechanical codes and tools necessary for this topic.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) Modelling, Nano, Materials

