Ab initio study on the kinetics of alkene isomerization and cracking in zeolite catalysis
Ab initio study on the kinetics of alkene isomerization and cracking in zeolite catalysis
Promotor(en): V. Van Speybroeck /16NANO03 / Nanoporous materialsCatalytic conversion of C4+ alkenes over zeolite catalysts is a widely applied technology in the (petro)chemical industry for the production of transportation fuels (gasoline, diesel, …) and base chemicals (light olefins, aromatics, paraffins, …). Alkene cracking often forms a crucial step in these processes and is therefore particularly relevant for both classical FCC processes as well as emerging alternatives, e.g., the methanol-to-olefins (MTO) process. Furthermore, olefin cracking will gain importance in so-called on-purpose ethene and propene producing processes due to the increasing imbalance between the light olefin demand and production. In these technologies, a considerable fraction of the primary products consists of heavier alkenes and further cracking of these species is necessary to achieve high overall ethene and propene yields. Given the importance of these reactions, a profound understanding of the cracking mechanism and kinetics is required to select or design the optimal performing catalyst for industrial FCC and MTO plants.
Alkene cracking occurs through a complex reaction network consisting of a multitude of hydride transfer, oligomerization, isomerization and β-scission (cracking) reactions. Consequently, a large pool of hydrocarbons with different length and branching will be formed inside the zeolite pores. A carbenium ion mechanism is generally accepted for the cracking reactions. However, due to the high reactivity of the reaction intermediates, even at lower temperatures, detailed information on the individual reaction steps in the network can hardly be obtained from experiments. Theoretical simulations can provide fundamental insights at the molecular level and aid in understanding experimental observations.
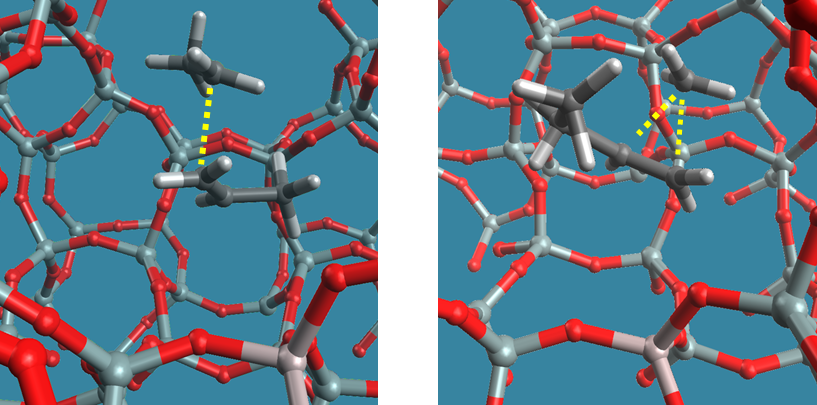
Figure 1. beta-scission (left) and isomerization (right) of a pentyl carbenium ion in H-ZSM-5
Goal
In this master thesis, the kinetics of alkene cracking in H-ZSM-5 will be studied. Different isomerization and cracking steps (see Figure 1) of selected alkenes in the C4 – C8 range will be investigated with advanced molecular modeling techniques. The goal is to identify the prevailing intermediates at relevant industrial process conditions and to elucidate the pathways leading to the desired products, i.e., ethene and propene. To this end, static calculations using accurate, contemporary functionals are in a first step combined with molecular dynamics simulations to account for the conformational freedom of the guest molecules. Next, you will use state-of-the-art rare-event sampling methods to unravel the underlying mechanisms and to predict the formation rates of the olefin products. Finally, the influence of temperature, branching degree, acidity, etc. will be assessed.
The Center for Molecular Modeling has ample experience in modeling of zeolite catalysis with advanced simulation techniques. The student will be actively coached to get acquainted with the multitude of available techniques to tackle the proposed problem. This thesis proposal will be conducted in close collaboration with prominent experimental partners in the field, providing an ideal opportunity to acquire valuable input and to validate the obtained results. The proposed topic is challenging and requires technical skills, creativity and chemical insight. The CMM will provide the necessary, extensive computational resources for studying this complex system.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsZeolites, Heterogeneous Catalysis, Chemical kinetics, Computational applicationsRecommended coursesMoleculaire modellering van industriële processen, Simulations and Modeling for the Nanoscale

