Nanofibers for drug delivery: Modelling Polymer-Drug interaction, how to obtain the most stable yet most drug releasing formula.
Nanofibers for drug delivery: Modelling Polymer-Drug interaction, how to obtain the most stable yet most drug releasing formula.
Promotor(en): V. Van Speybroeck, K. De Clerck /25389 / Chemistry & BiochemistryBackground and problem
With over 2/3rds of all drugs in development being classified as poorly water-soluble, it can be said that the pharmaceutical oral drug delivery industry is up for a challenge. Low water-solubility results in a low bioavailability of these active pharmaceutical ingredients, APIs, rendering them useless. As the main reason for this low water-solubility is the high crystallinity of the APIs, extensive research is being put into circumventing the crystallinity by obtaining and maintaining the API in its amorphous solid state. This is generally done through the use of amorphous solid dispersions of the API in an polymeric matrix. Several techniques such as spray drying or hot melt extrusion have already succeeded in obtaining such a solid dispersion, however the biggest obstacle is its time stability. As the amorphous state is essentially a meta-stable phase, current formulations have a tendency to recrystallize over time.
Because of this reason the solvent electrospinning technique is looked at to produce such polymer-drug amorphous solid dispersions. Because of the extremely rapid solvent evaporation during the solvent electrospinning process, it is believed that the API is frozen in a random and highly homogeneous manner in the polymeric matrix. Another advantage of the solvent electrospinning technique is that it creates nanofibrous membranes, which exhibit some unique and highly beneficial properties. The most important one being the very high specific surface, which allows for a faster dissolution rate.
Yet, one of the most important and least studied factors in making an efficient amorphous solid dispersion is by selecting the right polymer for the API. In order to have an enhanced bioavailability the drug needs to remain amorphous during storage and released when swallowed. In other words, the interaction between polymer and API is a crucial parameter. Sufficient interaction is required to guarantee time stability, however the strength of the interaction should also be limited in order to allow for the highest possible API release upon swallowing.
It is very hard to obtain insight into the molecular interactions from experimental point of view. In this sense molecular modeling may provide complementary insight as this allows to gain deep molecular insights into the interactions at working conditions, i.e. the aqueous environment, temperature, pressure and the applied polymers and API’s. Deep insight into the nanoscale interactions may guide the experiments towards a better polymer and API design. Such information on interactions also allows to enhance our chemical technological knowledge, e.g. on the meso-scale regarding multi-scale design of polymer based processes.
Goal
The goals of this master topic is to model investigate the crystallization behavior of various API’s in the presence of oxazoline nanofibers. To this end we first aim at a thorough understanding of the interaction types between different API’s and oxazoline (co-)polymers During the thesis we will try to gain fundamental understanding of the interaction types and the behavior of the API’s in an aqueous environment both in the presence and the absence of the polymers. The relevant API’s which are tested in the lab will also function as model compounds. These will be paired with biocompatible polymers such as poly(2-alkyl-2-oxazoline)s. The results of the models can eventually be experimentally validated and supported.
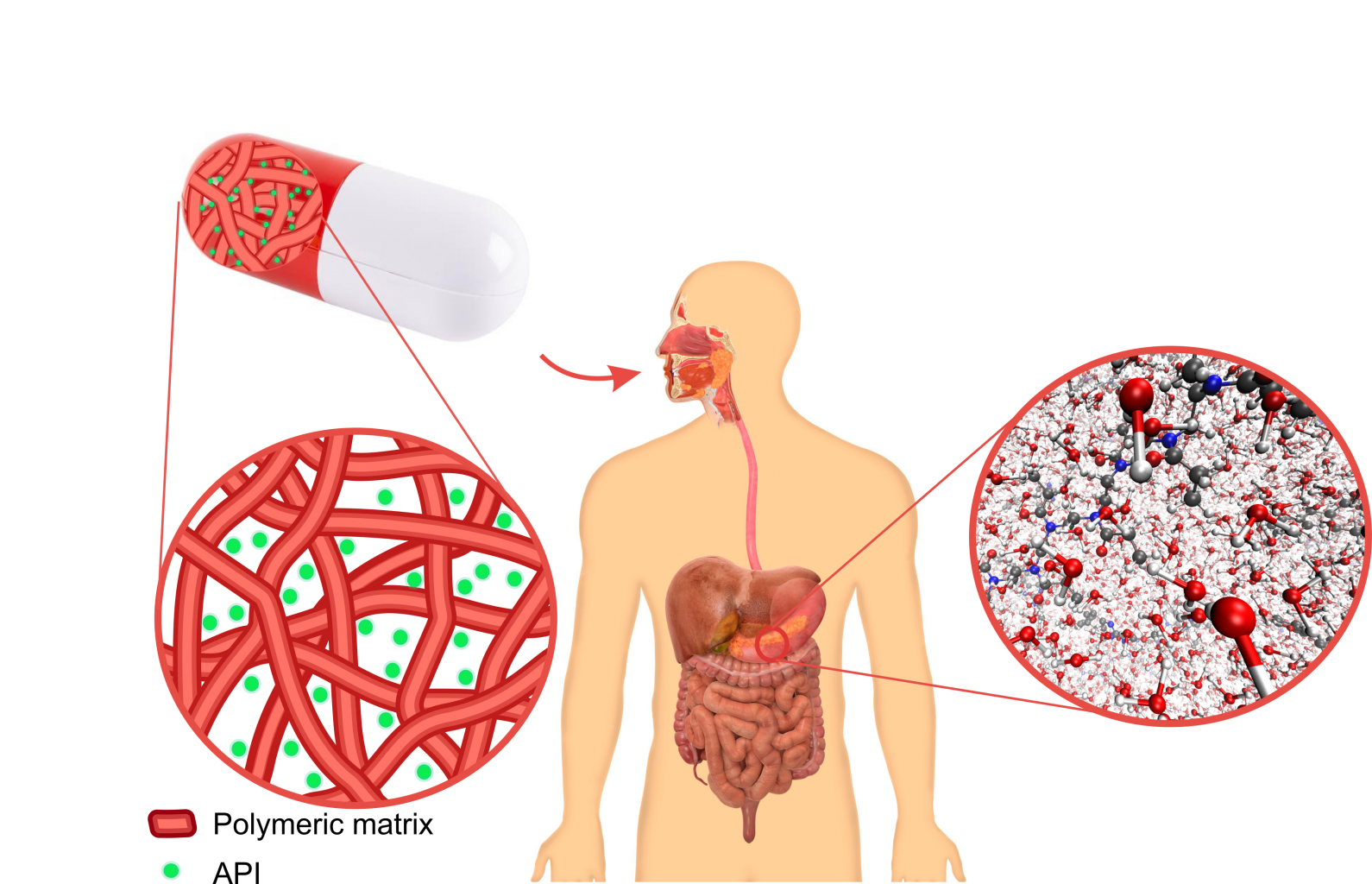
To obtain these goals, a multilevel modeling approach will be followed, Both Density functional theory (DFT) level calculations are performed to accurately describe the governing API-polymer and API-API interactions. These techniques solve the quantum mechanical electronic structure problem. At this level small model systems will be used for the polymers. Simultaneously, the student will use recently developed forcefield models to investigate the API-polymer systems in its true environment, mimicking the biological conditions of the stomach. These models hence allow us to follow the interactions at play at more realistic time scales, which can reach the nanosecond timescale while accounting for a more realistic environments (in contrast to DFT).
Finally the multilevel approach will allow to obtain a deep insight into the molecular configurations of the fiber, the API and solvent and to pinpoint the important interactions which are important use the APIs in realistic conditions. Additionally, there is a possibility of linking the theoretically found results to experimental results. By preparing and analyzing API-polymer solid dispersion as used in the models, the results can be experimentally validated
The calculations will be performed at the Center for molecular modeling with available software packages. The student will be actively coached to get familiar with the modeling programs. The calculations will performed on the high-performance computing facilities of the UGhent in combination with state of the art forcefield models and GPU accelerate codes. The CMM has already expertise in modeling poly-oxazolines.1–3 Furthermore the research group of Prof. Karen De Clerck has an active collaboration with the Center for molecular modeling, to unravel interactions between dyes in solvents.
- Study programmeMaster of Science in Biomedical Engineering [EMBIEN], Master of Science in Chemical Engineering [EMCHEM], Master of Science in Sustainable Materials Engineering [EMMAEN], International Master of Science in Biomedical Engineering [EMBIME]KeywordsNanofibers, active pharmaceutical ingredients, operando molecular modelling, Model development, amorphous solid dispersions, dissolution ratesReferences
(1) Goossens, H.; Catak, S.; Glassner, M.; de la Rosa, V. R.; Monnery, B. D.; De Proft, F.; Van Speybroeck, V.; Hoogenboom, R. ACS Macro Lett. 2013, 2 (8), 651–654.
(2) Bouten, P. J. M.; Hertsen, D.; Vergaelen, M.; Monnery, B. D.; Boerman, M. A.; Goossens, H.; Catak, S.; Van Hest, J. C. M.; Speybroeck, V. Van; Hoogenboom, R. Polym. Chem. 2014, 6, 514–518.
(3) Bouten, P. J. M.; Hertsen, D.; Vergaelen, M.; Monnery, B. D.; Catak, S.; van Hest, J. C. M.; Van Speybroeck, V.; Hoogenboom, R. J. Polym. Sci. Part A Polym. Chem. 2015, 53 (22), 2649–2661.
